Essential Thrombocythemia (ET): Treatment Approaches- Market Insights, Clinical Trials, Product Analysis, Patent Analysis, Competitive Analysis and Market Forecast – 2023-2033
Essential Thrombocythemia (ET) is a rare blood disorder characterized by an overproduction of platelets in the bone marrow, posing a risk of thrombus formation and severe complications such as strokes, heart attacks, or pulmonary embolisms. As a distinctive member of myeloproliferative neoplasms (MPNs), ET arises when bone marrow cells responsible for blood cell production exhibit abnormal development and function.
Ensuring an optimal prognosis for individuals with ET hinges on vigilant monitoring and timely interventions. While most can expect a normal life expectancy, a minority may experience a progression to Myelofibrosis, Acute Myeloid Leukemia, or, less frequently, Myelodysplastic syndrome.
Characteristics of Essential Thrombocythemia:
Symptoms, often absent in cases of elevated platelet levels, surface when related to blood clots. These may include headaches, confusion, chest pain, shortness of breath, nausea, weakness, and burning pain in the extremities.
Understanding the Causes:
ET is distinct from reactive thrombocytosis, arising from underlying medical issues like blood loss, cancer, infections, or inflammatory disorders. Reactive thrombocytosis typically resolves upon addressing the root cause. In contrast, the etiology of ET remains unclear but appears linked to genetic changes, leading to abnormal platelet production and functionality, elevating the risk of clotting or bleeding complications.
Complications and Diagnostic Measures:
ET can precipitate life-threatening consequences, including strokes, heart attacks, and, rarely, rapid-progressing leukemia. Diagnostic procedures involve a complete blood count (CBC), assessing platelet levels, iron levels, markers of inflammation, gene mutations, and, if required, a bone marrow biopsy.
Reactive Thrombocytosis:
Essential Thrombocythemia:
Revolutionary Drug Therapies:
Plateletpheresis:
Promising Clinical Trials: Ongoing research endeavors focus on advancing drug therapies, exploring innovative approaches to platelet management, and enhancing treatment options for ET, offering hope for improved patient outcomes and a brighter future in the landscape of blood disorder management.
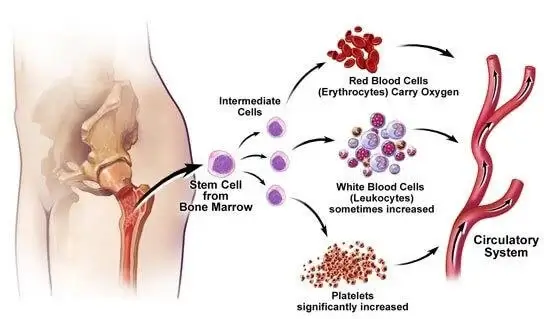
Figure 1 Essentially Thrombocythemia
Credits- (https://elink.io/p/et-essential-thrombocythemia-by-milka-99d112d)
To gain a comprehensive understanding of the evolving landscape in Essential Thrombocythemia (ET) research and development, it is essential to delve into the current state of scientific investigation and the emergence of innovative treatment strategies. This knowledge acts as a crucial guide for making well-informed decisions in the ET therapy arena, ensuring strategic choices and the effective incorporation of treatments within the evolving healthcare landscape for hematological disorders.
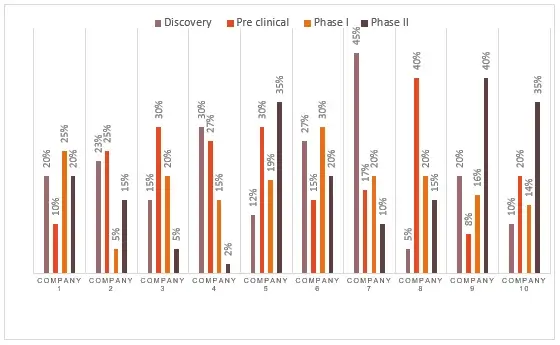
Figure 2 Distribution by Pipeline Candidates
In Essential Thrombocythemia (ET) research and market analysis, company profiling plays a crucial role. This entails a thorough investigation of companies involved in the ET sector, offering an in-depth view of a company’s historical background, product range, financial stability, competitive strategies, and recent achievements. This valuable information aids in evaluating the competitive landscape within the ET market, pinpointing potential opportunities for collaboration and strategic partnerships that can propel progress and innovation in the field.
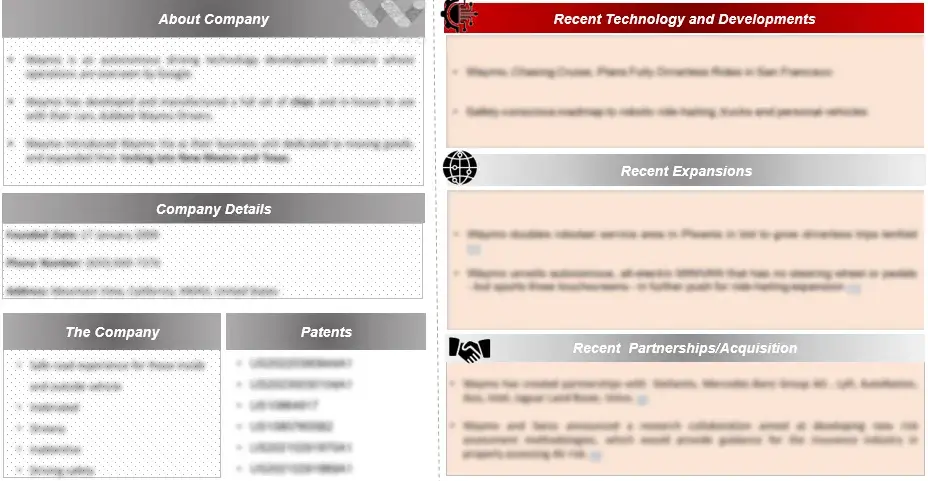
Figure 3 Company Profiles
Within this report, a comprehensive examination of patents will be carried out to thoroughly evaluate the intellectual property landscape in the Essential Thrombocythemia (ET) domain. The analysis seeks to unveil noteworthy patents, influential inventors, and emerging technological trends specific to ET. Moreover, it provides organizations with valuable insights into the realms of innovation, competition, and potential opportunities to enhance products or engage in collaborative ventures within the ET field.
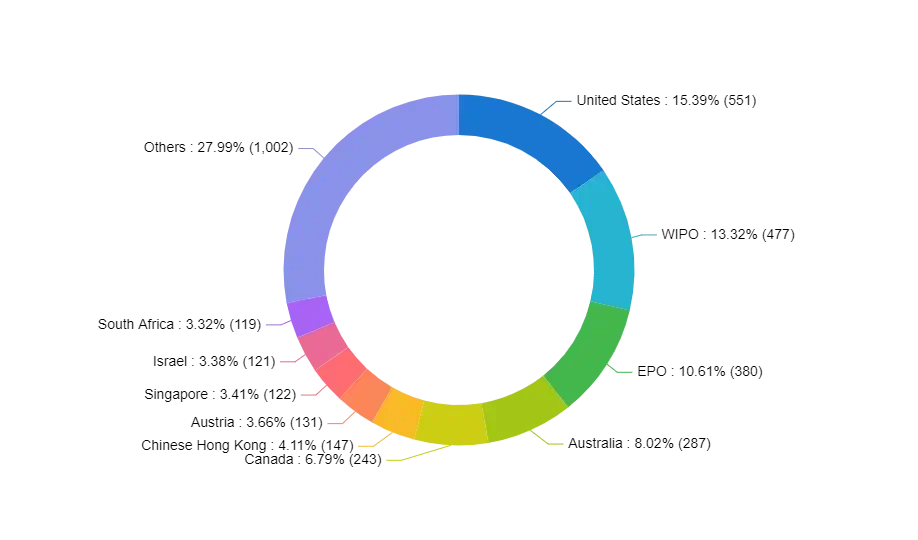
Figure 4 Geographic coverage of where patent applications have been filed
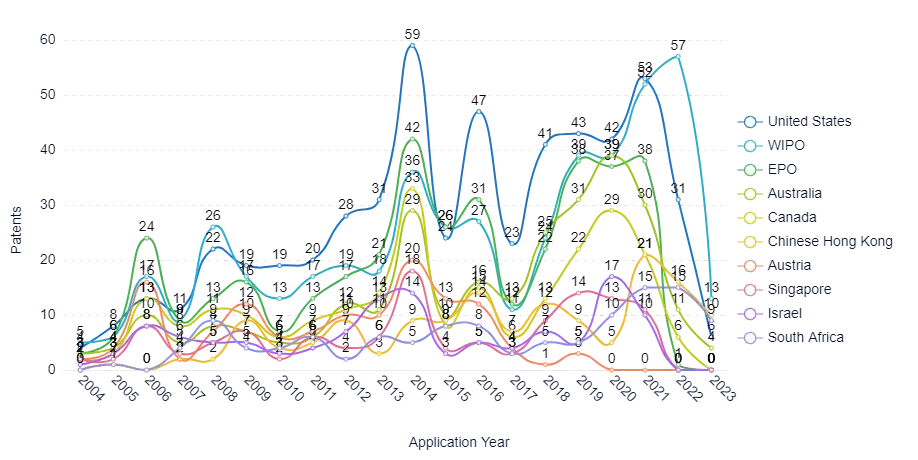
Figure 5 Yearly application trend of the Top Countries within the technology field
The clinical trial analysis section in a market research report offers a comprehensive evaluation of clinical trials related to Essential Thrombocythemia (ET). This includes a detailed breakdown of trial categories, study structures, research methods, and participant characteristics. Its main goal is to assess the effectiveness, safety, and outcomes of pharmaceuticals and medical interventions in the context of ET.
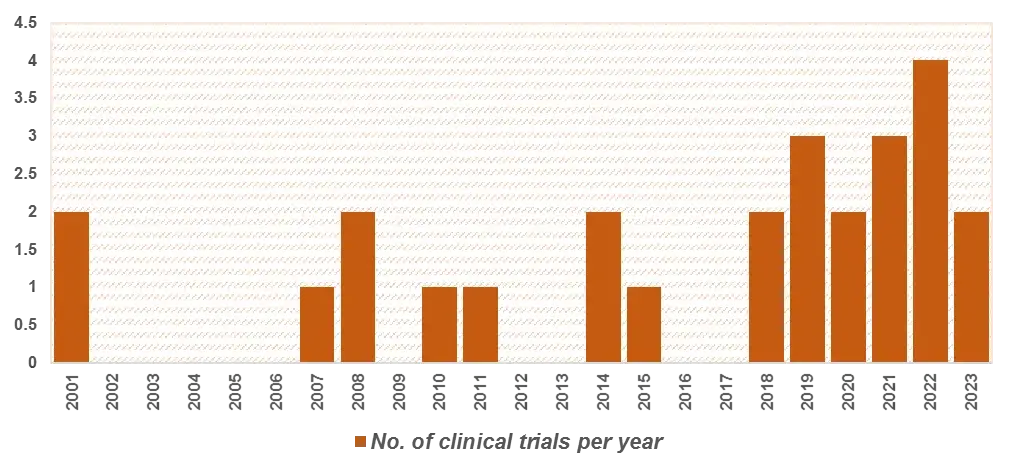
Figure 6 Clinical Trials Per Year
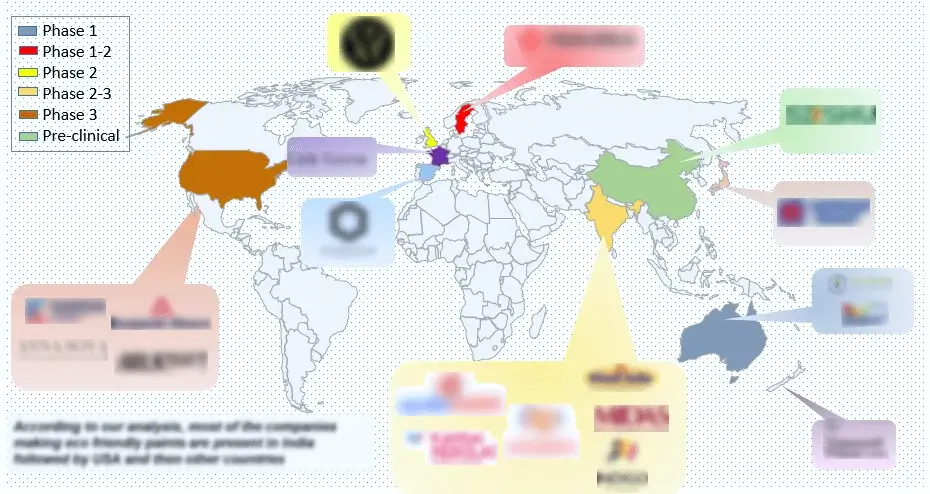
Figure 7 Distribution by Key Geographical Areas
Our exhaustive report provides valuable forecasts for Essential Thrombocythemia treatments, serving as a pivotal resource for stakeholders and industry experts. Through meticulous analysis of current trends, research progress, and emerging therapies, our report offers a forward-looking outlook on the evolving Essential Thrombocythemia treatment landscape. We explore factors like demographic shifts, regulatory updates, and the competitive scenario, facilitating informed decision-making for pharmaceutical companies, healthcare providers, and investors.
Figure 8 Essential Thrombocythemia (ET): Market, 2023-2033 (USD Million)
(Segmented in terms of the financial growth)
Within this market research report centered on Essential Thrombocythemia (ET), the section dedicated to the competitive landscape will offer a brief summary of key market participants. It will include details on their respective market shares and provide concise profiles outlining their strengths, weaknesses, and strategic approaches within the ET domain.
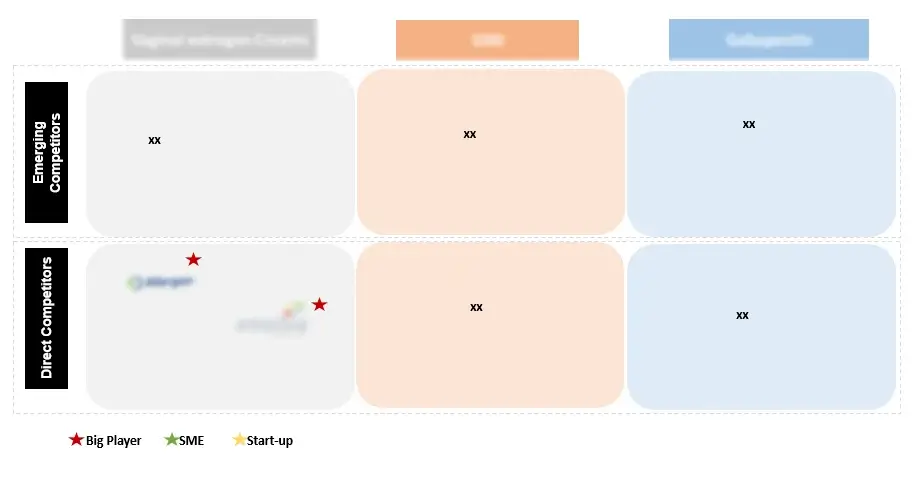
Figure 9 Competitive Landscape
Report Highlights:
Report Insights:
In order to give the most precise estimations and forecasts, Wissen Research uses an extensive and iterative research approach that is focused on reducing deviation. The company blends top-down and bottom-up methodologies for market segmentation and quantitative estimation. In addition, data triangulation, which examines the market from three separate angles, is a recurrent topic present in all of our research studies. Important components of the approach used for all of our studies include the following:
Preliminary data mining
On a wide scale, unprocessed market data is collected. Continuous data filtering makes sure that only verified and authenticated sources are taken into account. Additionally, data is extracted from a wide range of reports in our repository and from a number of reputable premium databases. We gather information from raw material suppliers, distributors, and purchasers to help with this since understanding the entire value chain is crucial for a thorough understanding of the market.
Surveys, technical symposia, and trade magazines are used to gather information on technical concerns and trends. Technical information focusing on white space and freedom of movement is also obtained from an intellectual property standpoint. Additionally, information on the industry’s drivers, constraints, and pricing patterns is obtained. As a result, a variety of original data are included in the material that is then cross-validated and certified with published sources.
Statistical model
We use simulation models to generate our market projections and estimates. Every study receives a special model that is tailored to it. Data for market dynamics, the technology environment, application development, and pricing patterns are gathered and supplied into the model all at once for analysis. The relative relevance of these factors is investigated, and their impact on the forecast period is assessed, using correlation, regression, and time series analysis. The process of market forecasting combines technological analysis with economic strategies, practical business acumen, and subject expertise.
Econometric models are frequently used for short-term forecasting, but technology market models are typically employed for long-term forecasting. These are based on a confluence of the business environment, regulatory environment, economic projection, and technical landscape. In order to develop global estimates, it is preferable to estimate markets from the bottom up by integrating data from key regional markets. This is required to ensure accuracy and a complete comprehension of the subject. Among the variables taken into account for forecasting are:
Regulations and anticipated developments
We give these criteria weights and use weighted average analysis to assess their market influence in order to calculate the anticipated market growth rate.
Primary research | Secondary research |
· Manufacturers · Technology distributors and wholesalers · End-user surveys · Consumer surveys | · Company reports and publications · Government publications · Independent investigations · Economic and demographic data · Online searches · Research reviews · Reference customers |
1. Executive Summary
1.1 Key Findings
1.2 Market Overview
1.3 Market Outlook
2. Introduction and Essential Thrombocythemia (ET) Overview
2.1 Neurodegenerative Disorders: An Overview
2.2 Scope of the Report
2.3 Research Methodology
2.4 Essential Thrombocythemia (ET): Understanding the Landscape
2.5 Classification and Characteristics of Essential Thrombocythemia
2.6 Disease Progression and Complications
3. Market Insight
3.1 Disease Prevalence and Epidemiology
3.2 Risk Factors and Etiology
3.3 Market Drivers
3.4 Market Challenges
3.5 Market Opportunities
3.6 Regulatory Landscape
4. Clinical Trial Analysis
4.1 Current Treatment Landscape
4.2 Emerging Therapies and Pipeline Analysis
4.3 Clinical Trial Design and Phases
4.4 Key Clinical Trials and Results
4.5 Future Prospects in ET Research
5. Product Analysis
5.1 Approved ET Drugs
5.2 Drug Classes and Mechanisms of Action
5.3 Emerging Therapies and Drug Candidates
5.4 Treatment Guidelines and Best Practices
5.5 Market Share Analysis of Leading Products
5.6 Product Analysis through Graphical Representation
5.7 Product Pipeline Analysis
5.8 Company Segmentation
5.9 Key Insights
6. Patent Analysis
6.1 Key Patents and Patent Holders
6.2 Patent Trends in ET Research
6.3 Patent Analysis by Geographical Regions
6.4 Top Assignees
6.5 Assignee Segmentation
6.6 Geographical Distribution of Top Assignees
6.7 Filing Trends
6.8 Litigations
6.9 Legal Status
7. Competitive Analysis
7.1 Market Players Overview
7.2 Benchmarking for Top Players
7.3 Company Profiles for Top 5 Players
7.4 SWOT Analysis for Top 5 Players
7.5 Competitive Strategies
7.6 Market Consolidation Trends
7.7 Emerging Market Trends
7.8 Future Outlook and Opportunities
7.9 Investment and Market Entry Strategies
8. Key Opinion Leaders (KOLs)
8.1 Detailed Analysis Identifying KOLs
8.2 Shortlisting Based on Contributions
9. Global ET Diagnostics Market, by End User
9.1 Overview
9.2 Hospitals
9.3 Diagnostic Centers
9.4 Pharmaceutical Companies
9.5 Clinics
10. Global Essential Thrombocythemia (ET) Diagnostics Market, By Diagnostic Test
10.1 Overview
10.2 Genetic Testing
10.3 Complete Blood Count (CBC)
10.4 Bone Marrow Examination
10.5 JAK2 Mutation Test
10.6 Additional Biomarker Tests
10.7 Imaging Studies
11. Global ET Diagnostics Market, By Geography
11.1 USA
11.2 Canada
11.3 Germany
11.4 U.K
11.5 France
11.6 Italy
11.7 Spain
11.8 Japan
12. Market Forecast – 2023-2033
12.1 Market Size and Growth Projections
12.2 Market Segmentation
12.3 By Treatment Type
12.4 By Region
12.5 By End User
13. Key Developments
13.1 Product Launches/Developments
13.2 Mergers and Acquisitions
13.3 Business Expansions
13.4 Partnerships and Collaborations
14. Conclusion
15. Appendix
15.1 Glossary of Terms
15.2 List of Abbreviations
15.3 Methodology
15.4 Data Sources
S.no | Key Highlights of Report | |
1. | Patent Analysis | · Top Assignee · Geography focus of top Assignees · Assignee Segmentation · Network analysis of the top collaborating entities in Essential Thrombocythemia (ET) therapy patent applications · Technology Evolution · Key Patents · Application and Issued Trend · Key technology |
2. | Market analysis | · Current Treatment Options · Emerging Therapies and Research Developments (by product analysis and scientific analysis) · Strategic activities · Therapeutic activity of drugs · Company portfolio · Detailed profiles of the key players that are engaged in the development of approved drugs |
3. | Clinical Trials | · Analysis of clinical trial through graphical representation · Coverage of treatments from pre-clinical phases till commercialization (also including terminated and completed studies) |
4. | Forecast | · Detailed comprehension of the historic, current and forecasted trend of market by analysis of impact of these treatments on the market |
5. | Opportunity Analysis | · Technology evolution based on problem solution · Potential licensees · Geography of suppliers · Treatment trends · Unmet needs · SWOT · Drivers and barriers |
6. | KOLs | · A detailed analysis and identification of the key opinion leaders (KOLs), shortlisted based on their contributions |
LIST OF FIGURES
Figure number | Description |
Figure 1 | Terminology of Essential Thrombocythemia (ET) Over The Years |
Figure 2 | Essential Thrombocythemia (ET) Treatment– History and Present |
Figure 3 | Projection of Essential Thrombocythemia (ET) till 2033 in different geographies |
Figure 4 | Technology Categorization Of Drug Delivery Methods For Essential Thrombocythemia (ET) |
Figure 5 | Recent Technology Trends in Essential Thrombocythemia (ET) |
Figure 6 | Technology Evolution in Drug Delivery Market of Essential Thrombocythemia (ET) |
Figure 7 | Geographical Distribution of Patents of Top Assignees |
Figure 8 | Assignee Segmentation (Companies) |
Figure 9 | Assignee Segmentation (Educational Establishment) |
Figure 10 | Patent Based Key Insights of xx |
Figure 11 | Patent Based Key insights of xx |
Figure 12 | Patent Based Key insights of xx |
Figure 13 | Geographic Distribution of the Universities/Research Organizations Filling Patents On Various Drug Delivery Approaches |
Figure 14 | Key Summary Regarding the Patent Filing On Essential Thrombocythemia (ET) |
Figure 15 | Product Pipeline of Different Approaches with Companies Name |
Figure 16 | Portfolio for Approved Product |
Figure 17 | Clinical Trials Conducted till Date by Different Companies and Universities |
Figure 18 | Clinical Trials based Key Insights |
Figure 19 | Key Growth Drivers for Essential Thrombocythemia (ET) Market |
Figure 20 | Restraints for Essential Thrombocythemia (ET) Market |
Figure 21 | xx Portfolio (Top Player) |
Figure 22 | xx Portfolio (Top Player) |
Figure 23 | xx Portfolio (Top Player) |
Figure 24 | xx Portfolio (Top Player) |
Figure 25 | xx Portfolio (Top Player) |
Figure 26 | xx Portfolio (Start-up) |
Figure 27 | xx Portfolio (Start-up) |
Figure 28 | xx Portfolio (Start-up) |
Figure 29 | Strategic Activities Including Collaboration, Partnerships and Acquisitions |
Figure 30 | Research Methodology for Patent, Selection and Analysis |
Figure 31 | Research Methodology for Clinical Trials, Selection and Analysis |
LIST OF GRAPHS
Graph number |
Description |
Graph 1 | Number of people worldwide with Essential Thrombocythemia (ET) |
Graph 2 | Problem Solution Analysis |
Graph 3 | Top Assignees in Essential Thrombocythemia (ET) |
Graph 4 | Technology Focus of Top Assignees (IPC-CPC Classes) |
Graph 5 | Top Countries of Origin of Patents |
Graph 6 | New entrants in drug delivery field |
Graph 7 | Legal Status |
Graph 8 | Most Cited Patents |
Graph 9 | Patents with Largest Invention Families |
Graph 10 | Most Claim-Heavy Patents |
Graph 11 | Filing Trends |
Graph 12 | Clinical Trial Filing Timeline |
Graph 13 | Recruitment Status of the Clinical Trials Related to the Different Drug Delivery Approaches |
Graph 14 | Clinical Trials Phases with Respect to Specific Drug Delivery Approach |
Graph 15 | Weighted Scores for Top 5 Players According to Benchmarking Criteria |
Graph 16 | Essential Thrombocythemia (ET) (CAGR: 2023-2033) |
Graph 17 | Essential Thrombocythemia (ET) Market Share: Distribution by Key Geographical Area, 2023-2033 |
LIST OF TABLES
Table number | Description |
Table 1 | Parameters included and excluded for conducting the analysis |
Table 2 | Technology Classes with Definitions |
Table 3 | Patent Litigation |
Table 4 | Highest Market Valued Patents |
Table 5 | SWOT Analysis of Top 3 Players |
Table 6 | Parameters and their score for Benchmarking |
Table 7 | Weighted scores for top 5 players according to benchmarking criteria |
© Copyright 2024 – Wissen Research All Rights Reserved.