Acute Kidney Injury (AKI), also known as acute renal failure, represents a clinical syndrome marked by a sudden and swift decline in kidney function, leading to disturbances in electrolyte balance, volume regulation, and the accumulation of nitrogenous waste. This condition, often observed in hospitalized patients and the elderly, necessitates prompt attention due to its potential impact on various organs, including the brain, heart, and lungs.
Diagnostic criteria for AKI involve a rise in serum creatinine levels by 0.3 mg/dL within 48 hours, a 1.5 times increase from baseline within the last seven days, or a urine output less than 0.5 mL/kg/hour for six hours. Early intervention is crucial as serum creatinine may not reflect kidney dysfunction until a substantial proportion of renal mass is damaged.
Treatment for AKI is primarily supportive, with an emphasis on addressing underlying causes. Therapeutic agents such as dopamine, nesiritide, fenoldopam, and mannitol have shown limited efficacy and may even be harmful. The mainstay of treatment involves maintaining volume homeostasis and correcting biochemical abnormalities. This includes managing fluid overload with furosemide, correcting severe acidosis with bicarbonate administration, and addressing hyperkalemia through various approaches like dietary modification and dialysis.
Nephrotoxic agents, including certain medications and chemicals, should be used cautiously or avoided altogether. A retrospective study revealed a heightened risk of AKI associated with triple therapy involving nonsteroidal anti-inflammatory drugs (NSAIDs) and two antihypertensive medications.
Vasodilator therapy, such as fenoldopam, has shown promise in reducing the need for renal replacement therapy and lowering mortality rates, but further research is needed for conclusive recommendations. Dopamine, once considered beneficial in renal vasculature dilation, lacks consistent evidence supporting its efficacy in AKI.
Dietary modification is a crucial aspect of AKI management, involving salt and fluid restriction, and careful monitoring of potassium and phosphorus levels. Dialysis, particularly hemodialysis, becomes necessary in cases of volume expansion, refractory hyperkalemia, severe acid-base disturbances, and when uremia is pronounced.
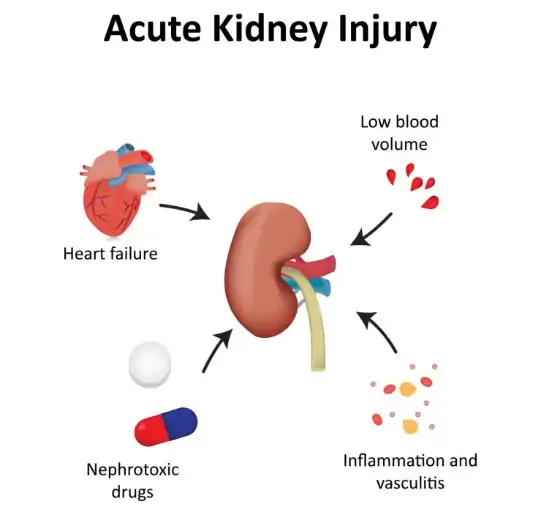
Figure 1 Credits: >>
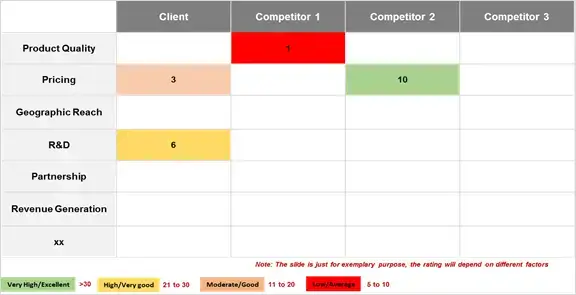
To effectively assess and position oneself in the competitive landscape of Acute Kidney Injury (AKI) over the short to mid-long term, it is essential to have a thorough understanding of the developmental pipeline for AKI. This requires staying abreast of the evolving landscape, competitor strategies, and expected advancements in the field.
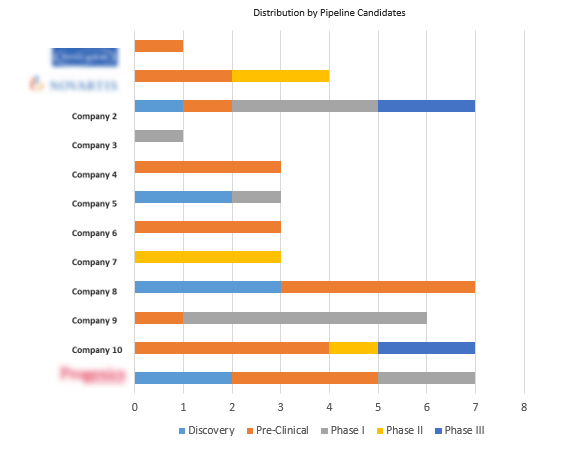
Figure 2 Ongoing Research
Profiles of key stakeholders propelling advancements in Acute Kidney Injury (AKI) solutions provide valuable insights into their financials, product portfolios, and recent innovations in the field.
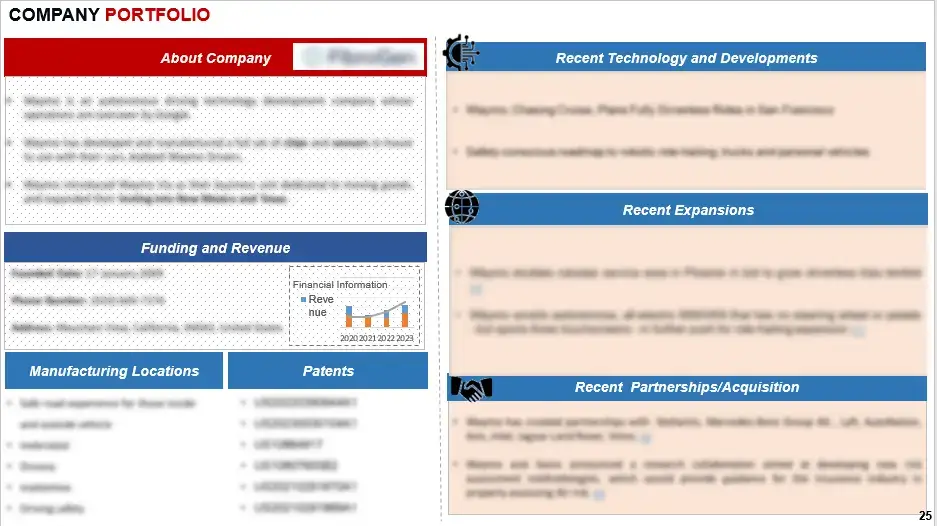
Figure 3 Company Portfolio
This report explores patents related to Acute Kidney Injury (AKI), uncovering crucial innovations and pinpointing opportunities for improvements or collaborations within the field.
Figure 4 Annual Patent Filing Trends
This report on Acute Kidney Injury (AKI) foresees trends, delineates growth opportunities, and offers crucial insights into market dynamics, encompassing aspects such as size, revenue forecasts, and growth potentials.
Figure 5 Yearly Market Growth, 2016-2024 (USD Billion)
(Segmented in terms of the financial growth)
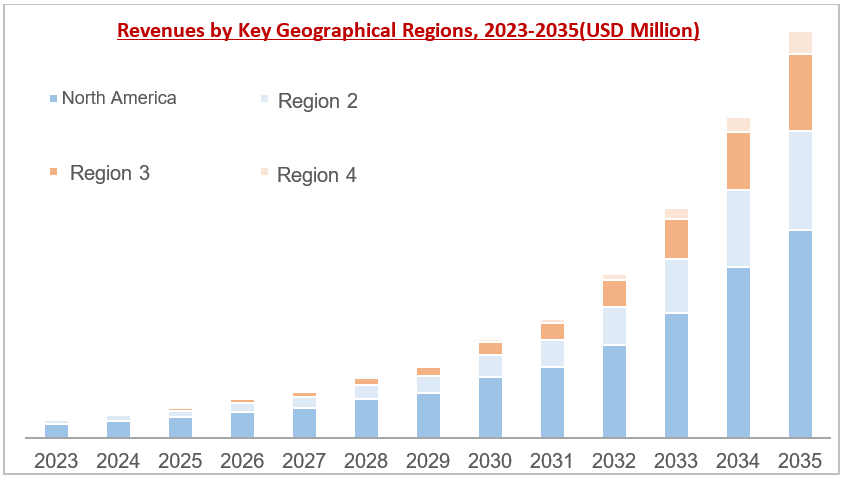
Figure 6 Forecast by Key Geographical Regions
This report on Acute Kidney Injury (AKI) spotlights prominent market players, presenting succinct profiles that delve into market shares, strengths, weaknesses, and strategic approaches.
In order to give the most precise estimations and forecasts, Wissen Research uses an extensive and iterative research approach that is focused on reducing deviation. The company blends top-down and bottom-up methodologies for market segmentation and quantitative estimation. In addition, data triangulation, which examines the market from three separate angles, is a recurrent topic present in all of our research studies. Important components of the approach used for all of our studies include the following:
On a wide scale, unprocessed market data is collected. Continuous data filtering makes sure that only verified and authenticated sources are taken into account. Additionally, data is extracted from a wide range of reports in our repository and from a number of reputable premium databases. We gather information from raw material suppliers, distributors, and purchasers to help with this since understanding the entire value chain is crucial for a thorough understanding of the market.
Surveys, technical symposia, and trade magazines are used to gather information on technical concerns and trends. Technical information focusing on white space and freedom of movement is also obtained from an intellectual property standpoint. Additionally, information on the industry’s drivers, constraints, and pricing patterns is obtained. As a result, a variety of original data are included in the material that is then cross-validated and certified with published sources.
We use simulation models to generate our market projections and estimates. Every study receives a special model that is tailored to it. Data for market dynamics, the technology environment, application development, and pricing patterns are gathered and supplied into the model all at once for analysis. The relative relevance of these factors is investigated, and their impact on the forecast period is assessed, using correlation, regression, and time series analysis. The process of market forecasting combines technological analysis with economic strategies, practical business acumen, and subject expertise.
Econometric models are frequently used for short-term forecasting, but technology market models are typically employed for long-term forecasting. These are based on a confluence of the business environment, regulatory environment, economic projection, and technical landscape. In order to develop global estimates, it is preferable to estimate markets from the bottom up by integrating data from key regional markets. This is required to ensure accuracy and a complete comprehension of the subject. Among the variables taken into account for forecasting are:
Regulations and anticipated developments
We give these criteria weights and use weighted average analysis to assess their market influence in order to calculate the anticipated market growth rate.
Primary research | Secondary research |
· Manufacturers · Technology distributors and wholesalers · End-user surveys · Consumer surveys | · Company reports and publications · Government publications · Independent investigations · Economic and demographic data · Online searches · Research reviews · Reference customers |
**not an exhaustive list
S.no | Key Highlights of Report | |
1. | Patent Analysis | · Top Assignee · Geography focus of top Assignees · Assignee Segmentation · Network analysis of the top collaborating entities in Acute Kidney Injury (AKI) therapy patent applications · Technology Evolution · Key Patents · Application and Issued Trend · Key technology |
2. | Market analysis | · Current Treatment Options · Emerging Therapies and Research Developments (by product analysis and scientific analysis) · Strategic activities (Collaboration/Mergers/Agreements/Partnerships/Acquisitions taken place by analyzed companies) · Therapeutic activity of drugs · Company portfolio |
3. | Clinical Trials | · Analysis of clinical trial through graphical representation · Coverage of treatments from pre-clinical phases till commercialization (also including terminated and completed studies) |
4. | Forecast | · Detailed comprehension of the historic, current and forecasted trend of market by analysis of impact of these treatments on the market · Drivers and barriers |
5. | Key Players | · Detailed profiles of the key players that are engaged in the development of approved drugs |
6. | Opportunity Analysis | · Technology evolution based on problem solution · Potential licensees · Geography of suppliers · Treatment trends · Unmet needs · Opportunity of new treatments |
7. | KOLs | · A detailed analysis and identification of the key opinion leaders (KOLs), shortlisted based on their contributions |
8. | SWOT | · SWOT analysis for treatments · Market Access |
LIST OF FIGURES
Figure number | Description |
Figure 1 | Terminology of Acute Intermittent Porphyria Over The Years |
Figure 2 | Acute Intermittent Porphyria Treatment– History and Present |
Figure 3 | Projection of Acute Intermittent Porphyria till 2033 in different geographies |
Figure 4 | Technology Categorization Of Drug Delivery Methods For Acute Intermittent Porphyria |
Figure 5 | Recent Technology Trends in Acute Intermittent Porphyria |
Figure 6 | Technology Evolution in Drug Delivery Market of Acute Intermittent Porphyria |
Figure 7 | Geographical Distribution of Patents of Top Assignees |
Figure 8 | Assignee Segmentation (Companies) |
Figure 9 | Assignee Segmentation (Educational Establishment) |
Figure 10 | Patent Based Key Insights Of xx |
Figure 11 | Patent Based Key insights of xx |
Figure 12 | Patent Based Key insights of xx |
Figure 13 | Geographic Distribution of the Universities/Research Organizations Filling Patents On Various Drug Delivery Approaches |
Figure 14 | Key Summary Regarding the Patent Filing On Acute Intermittent Porphyria |
Figure 15 | Product Pipeline of Different Approaches with Companies Name |
Figure 16 | Portfolio for Approved Product |
Figure 17 | Clinical Trials Conducted till Date by Different Companies and Universities |
Figure 18 | Clinical Trials based Key Insights |
Figure 19 | Key Growth Drivers for Acute Intermittent Porphyria |
Figure 20 | Restraints for Acute Intermittent Porphyria |
Figure 21 | xx Portfolio (Top Player) |
Figure 22 | xx Portfolio (Top Player) |
Figure 23 | xx Portfolio (Top Player) |
Figure 24 | xx Portfolio (Top Player) |
Figure 25 | xx Portfolio (Top Player) |
Figure 26 | xx Portfolio (Start-up) |
Figure 27 | xx Portfolio (Start-up) |
Figure 28 | xx Portfolio (Start-up) |
Figure 29 | Strategic Activities Including Collaboration, Partnerships and Acquisitions |
Figure 30 | Research Methodology for Patent, Selection and Analysis |
Figure 31 | Research Methodology for Scientific Literature, Selection and Analysis |
Figure 32 | Research Methodology for Clinical Trials, Selection and Analysis |
LIST OF GRAPHS
Graph number |
Description |
Graph 1 | Number of people worldwide with Acute Intermittent Porphyria |
Graph 2 | Problem Solution Analysis |
Graph 3 | Top Assignees in Acute Intermittent Porphyria |
Graph 4 | Technology Focus of Top Assignees (IPC-CPC Classes) |
Graph 5 | Top Countries of Origin of Patents |
Graph 6 | New entrants in drug delivery field |
Graph 7 | Legal Status |
Graph 8 | Most Cited Patents |
Graph 9 | Patents with Largest Invention Families |
Graph 10 | Most Claim-Heavy Patents |
Graph 11 | Filing Trends |
Graph 12 | Literature Filling Trend During Time Period (2018 – 2023) |
Graph 13 | Clinical Trial Filing Timeline |
Graph 14 | Recruitment Status of the Clinical Trials Related to the Different Drug Delivery Approaches |
Graph 15 | Clinical Trials Phases with Respect to Specific Drug Delivery Approach |
Graph 16 | Weighted Scores for Top 64 Players According to Benchmarking Criteria |
Graph 17 | Acute Intermittent Porphyria (CAGR: 2023-2033) |
Graph 18 | Acute Intermittent Porphyria Market Share: Distribution by Key Geographical Area, 2023-2033 |
LIST OF TABLES
Table number | Description |
Table 1 | Parameters included and excluded for conducting the analysis |
Table 2 | Technology Classes with Definitions |
Table 3 | Patent Litigation |
Table 4 | Highest Market Valued Patents |
Table 5 | SWOT Analysis of Top 3 Players |
Table 6 | Parameters and their score for Benchmarking |
Table 7 | Weighted scores for top 5 players according to benchmarking criteria |
© Copyright 2024 – Wissen Research All Rights Reserved.