Medical Device Contract Manufacturing Market by Service Type (Quality Management, Device Development, Packaging Services), Device Type (Diagnostic, Therapeutic, Drug Delivery Devices), Class (Class I, Class II, Class III), Target Application (Cardiovascular, Diabetes, Dental, Orthopedic, Ophthalmology, Respiratory, Surgical Applications), Regions (Asia Pacific, Europe, North America, Middle East and Africa, and Latin America), Key Players – Global Forecast to 2030
 Sources: Company Websites, Annual Reports, SEC Filings, Press Releases, Investor Presentation, Paid Database, and Wissen Research Analysis.
Sources: Company Websites, Annual Reports, SEC Filings, Press Releases, Investor Presentation, Paid Database, and Wissen Research Analysis.
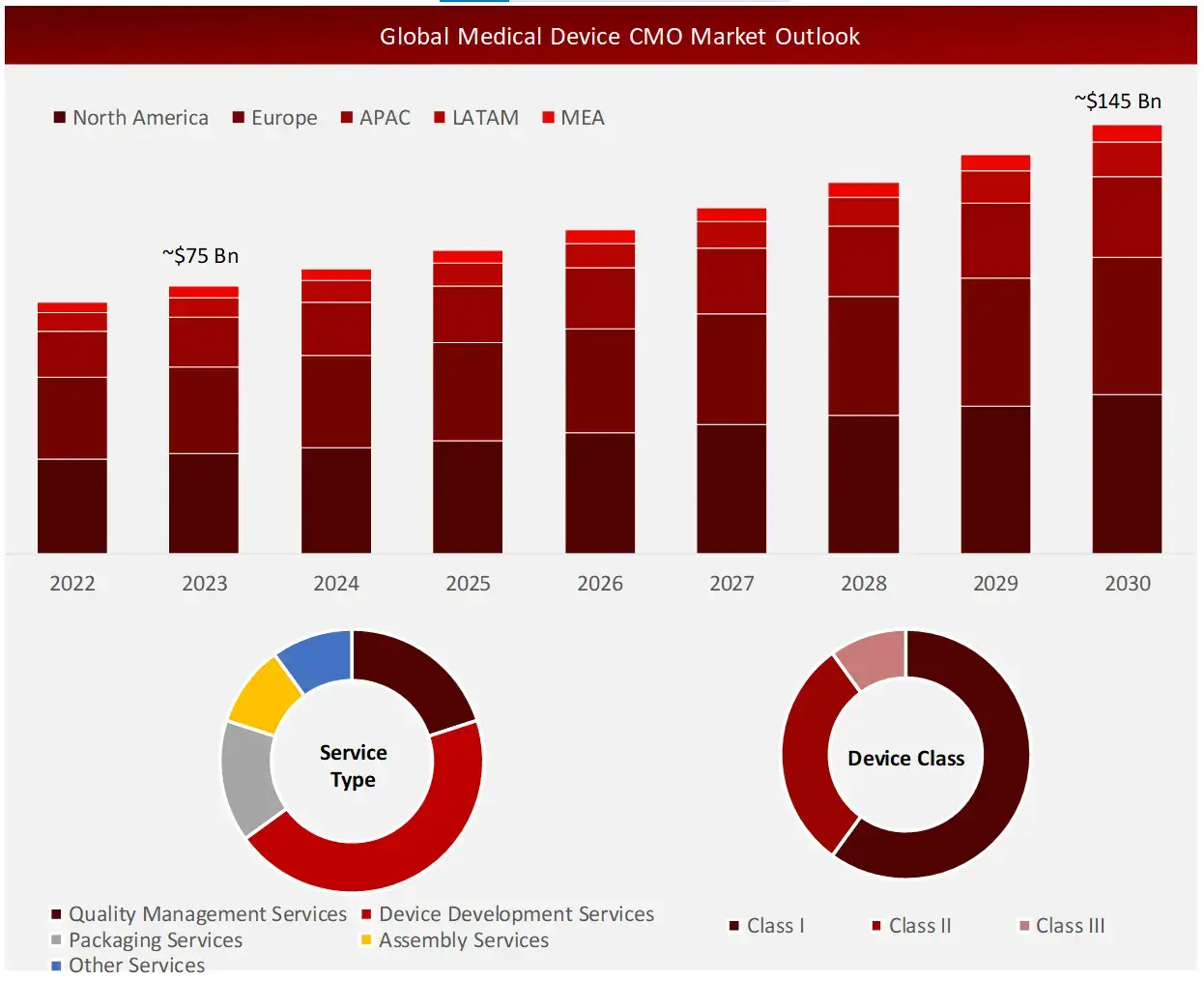
Wissen Research analyses that the global medical device CMO market is estimated at ~USD 75 billion in 2023 and is projected to reach ~USD 145 billion by 2030, expected to grow at a CAGR of ~11% during the forecast period, 2023-2030.
The increasing demand for medical devices in recent years, is pushing medical device developers to outsource parts of their product development and manufacturing to contract service providers. Contract manufacturers of medical devices are concentrating on strengthening their internal capacities in order to provide a broad range of services which include product design, distribution, marketing, and regulatory support, to their clients. According to BMP Medical, a medical device contract manufacturer, there are close to 5,800 active companies in medical device industry. It is worth mentioning that with the rising need for innovative devices, need for decreasing time to market and emphasis on regulatory compliance the medical device CMO sector is expected to play a vital role in offering outsourcing services, therefore, the demand for more medical CMOs will grow in the near future.
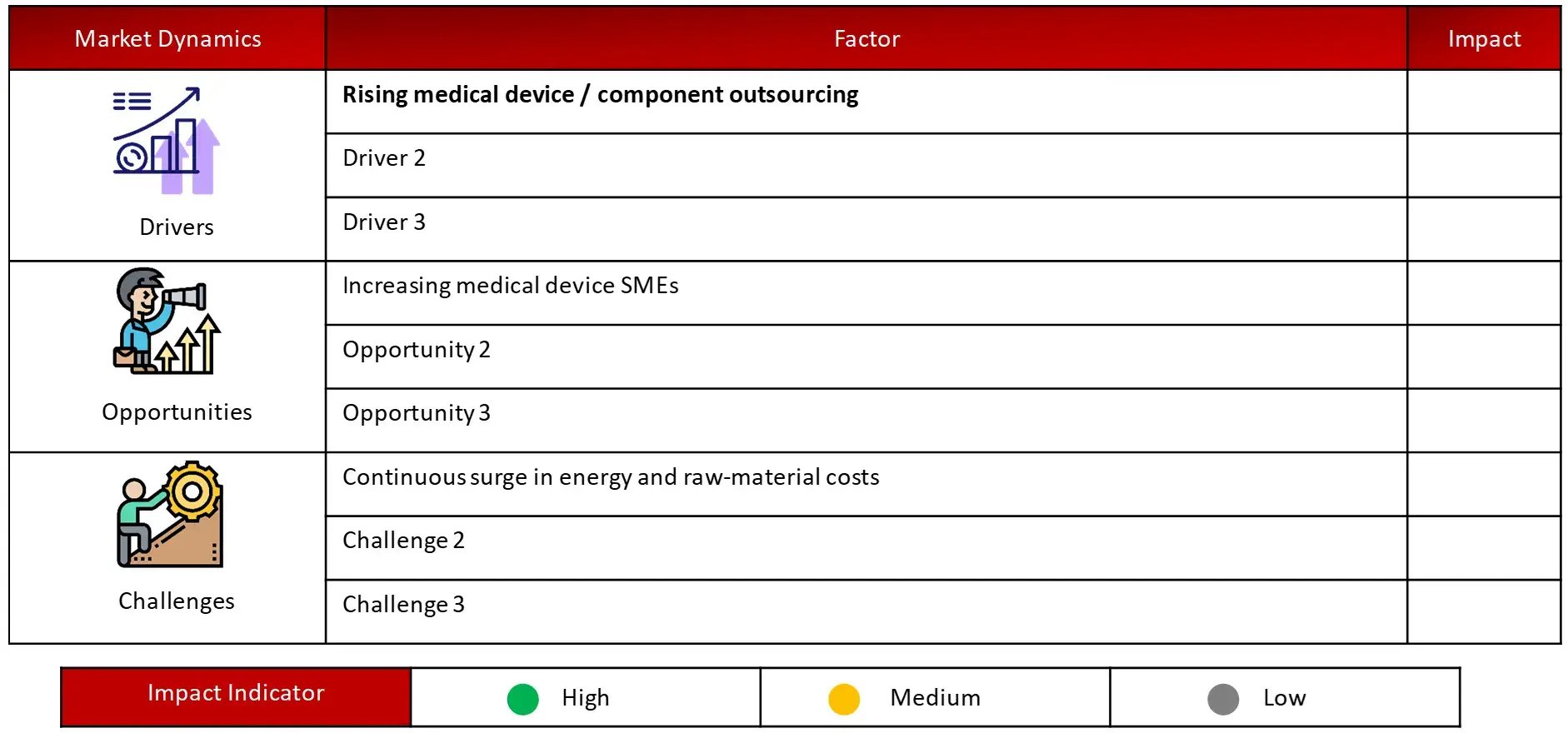
Driving Factor: Rising high-tech innovation and improved capabilities
Given the increasing adoption of advanced data science technologies and automation in the past few years, the medical device CMOs have been able to deliver high quality and precise medical devices to its customers. By early utilization and investments in advanced tools / methods, CMOs are anticipated to have competitive advantage, therefore, will meet the rising demand for innovative medical devices and components.
Opportunity: Increasing number of small and mid-sized medical device developers in emerging markets
Due to their cost advantages and favorable regulations, emerging regions such as, Asia Pacific, have become the suitable outsourcing hubs. Factors such as, low labor and manufacturing cost, increasing disposable income, are anticipated to offer discernible opportunities for the growth of medical device CMOs market in the near future.
Challenge: Growing raw material costs and stringent regulatory guidelines impacts growth and operations of CMOs
Rising cost, especially with fluctuations in raw material cost has become a constant challenge and thus pressurize CMOs in managing operational costs while maintaining quality and compliance. Further, with strict regulatory guidelines across various geographies, compliance to such regulations can be complex and time consuming.
The medical device CMO market report offers information on the latest advancements in the industry, as well as service portfolio analysis, supply chain/value chain analysis, market share, and the effects of localized and domestic players. It also analyzes potential revenue opportunities and changes in market regulations, as well as market size, category market growth, application dominance, product approvals, service launches, geographic expansions, and technological innovations. For an Analyst Brief and other information on the medical device CMO market, get in touch with Wissen Research. Our staff can assist you in making well-informed decisions that will lead to market expansion.
 Sources: Company Websites and Wissen Research Analysis.
Sources: Company Websites and Wissen Research Analysis.
Diagnostic devices are expected to hold largest rate within the medical CMO market by device type
The medical device CMO market is segmented into diagnostic devices, therapeutic devices, drug delivery devices and other devices. Presently, diagnostic devices segment is anticipated to hold majority share. This can be ascribed to the increasing demand for precision and early diagnostics of serious health problems. However, drug delivery devices are expected to witness highest growth rate in the coming decade.
Device Development Services captured majority share of current medical device CMO market by service type
Quality management services, device development services, packaging services, assembly services, and other services are the key segments that the medical device contract manufacturers are available in. In 2024, device development services segment captures the largest share out of all these services. This can be attributed to the fact that device design and development often requires highly expensive advanced technologies which medical device developers might be lacking for in-house development of medical devices and components.
Major players operating in medical device CMO market are Jabil Inc., SMC Ltd., Gerresheimer AG, Nipro Corporation, Flex Ltd., Nortec Systems, Inc., Kimball Electronics Inc., Peter’s Technology Ltd., TE Connectivity Ltd., and Integer Holdings Corporation among others.
Recent Developments:
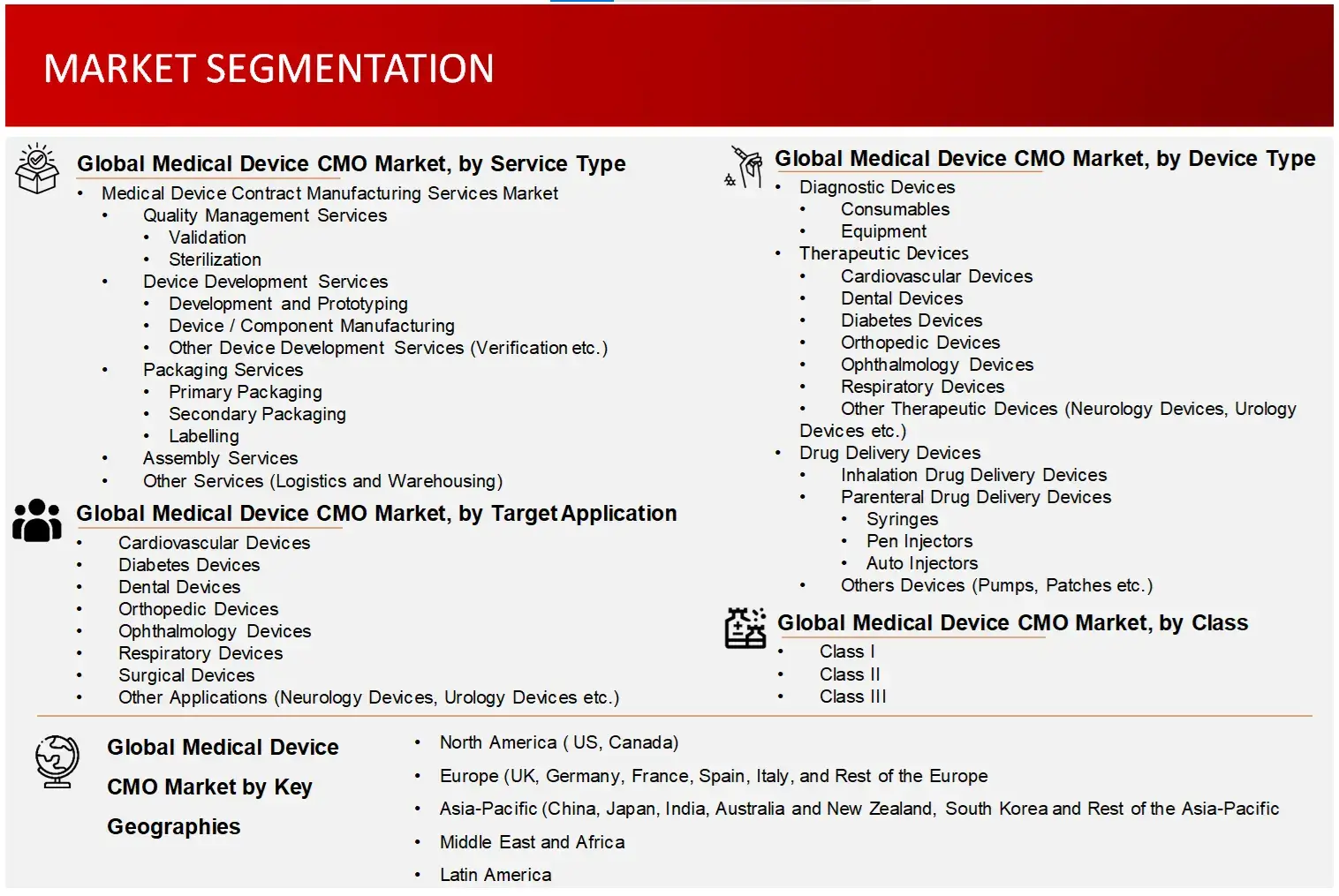
Market Definition
Medical Device Contract Manufacturing Organizations (CMOs) are third-party service providers, that handles manufacturing of medical diagnostic, imaging, therapeutic equipment and other complex systems of medical devices and components. CMOs offer significant cost-benefits, precision, access to sophisticated infrastructure, production efficiency, large development capacities and reduction in time-to-market for medical devices and components.

Key Stakeholders
Key objectives of the Study
Research Methodology
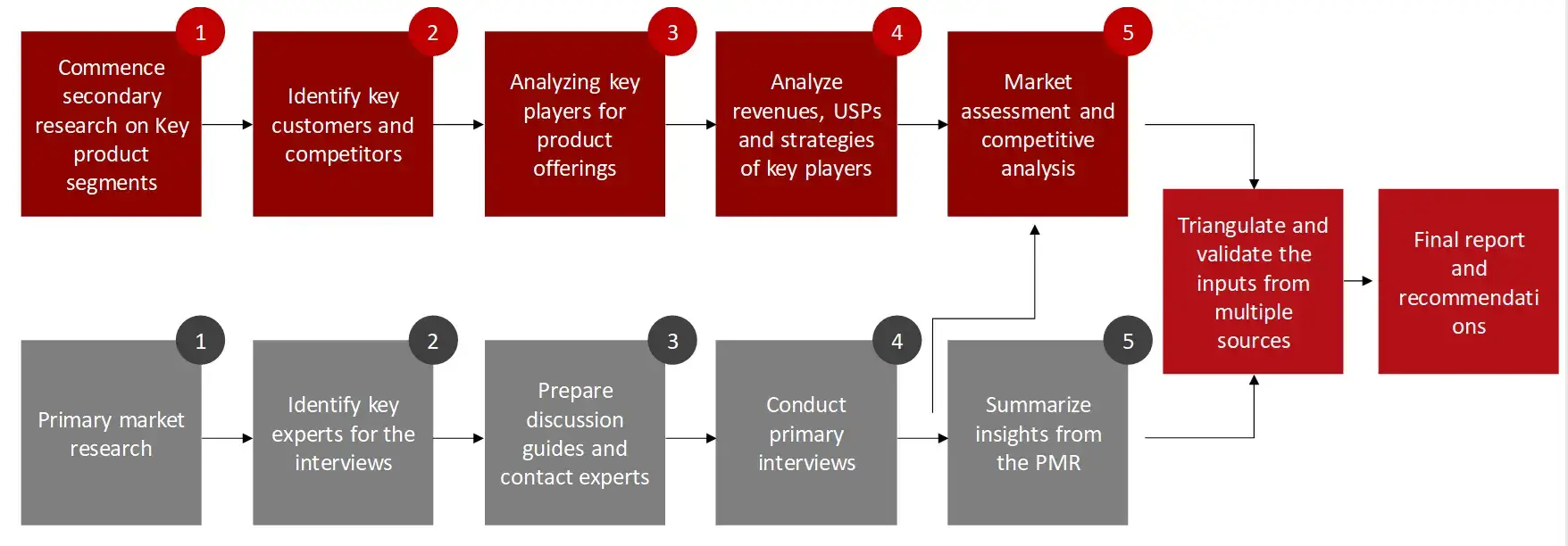
The aim of the study is to examine the key market forces such as drivers, opportunities, restraints, challenges, and strategies of key leaders. To monitor company advancements such as patents granted, product launches, expansions, and collaborations of key players, analyzing their competitive landscape based on various parameters of business and product strategy. Markey sizing will be estimated using top-down and bottom-up approaches. Using market breakdown and data triangulation techniques, market sizing of segments and sub-segments will be estimated.
FIGURE: RESEARCH DESIGN
 Sources: Wissen Research Analysis.
Sources: Wissen Research Analysis.Research Approach
Collecting Secondary Data
The process of collating secondary research data involves the utilization of databases, secondary sources, annual reports, investor presentations, directories, and SEC filings of companies. Secondary research will be utilized to identify and gather information beneficial for the in-depth, technical, market-oriented, and commercial analysis of the medical device CMO market. A database of the key industry leaders will also be compiled using secondary research.
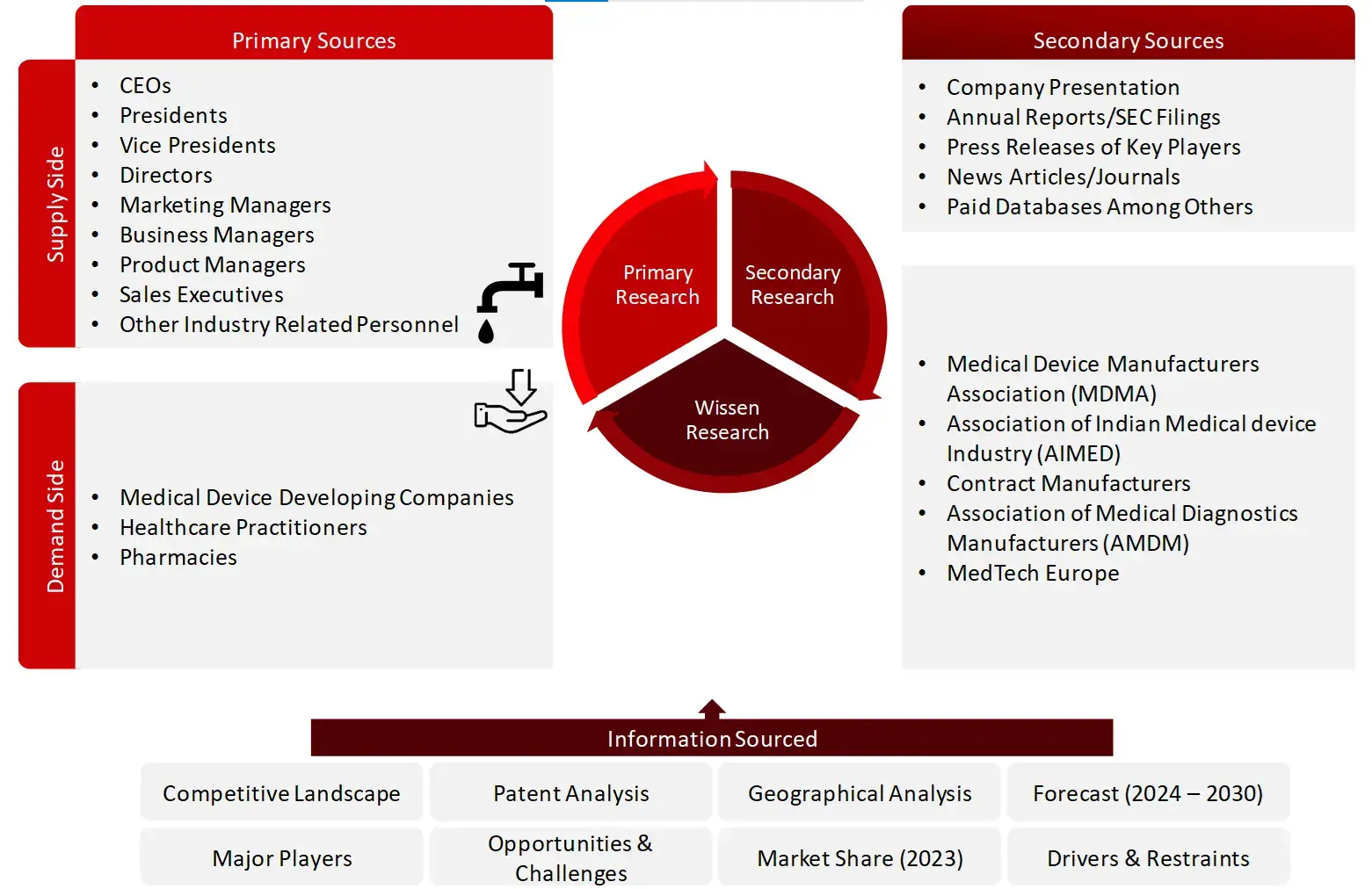
Collecting Primary Data
The primary research data will be conducted after acquiring knowledge about the medical device CMO market scenario through secondary research. A significant number of primary interviews will be conducted with stakeholders from both the demand and supply side (including various industry experts, such as Vice Presidents (VPs), Chief X Officers (CXOs), Directors from business development, marketing and product development teams, product manufacturers) across major countries of Europe, Asia Pacific, North America, Latin America, and Middle East and Africa. Primary data for this report will be collected through questionnaires, emails, and telephonic interviews.
FIGURE: BREAKDOWN OF PRIMARY INTERVIEWS FROM SUPPLY SIDE
FIGURE: BREAKDOWN OF PRIMARY INTERVIEWS FROM DEMAND SIDE
FIGURE: PROPOSED PRIMARY PARTICIPANTS FROM DEMAND AND SUPPLY SIDE
 Note: Above mention companies are non-exhaustive.
Note: Above mention companies are non-exhaustive.
Market Size Estimation
All major manufacturers offering various medical device CMO services will be identified at the global / regional level. Revenue mapping will be done for the major players, which will further be extrapolated to arrive at the global market value of each type of segment. The market value of medical device CMO market will also split into various segments and sub segments at the region level based on:
FIGURE: REVENUE MAPPING BY COMPANY (ILLUSTRATION)
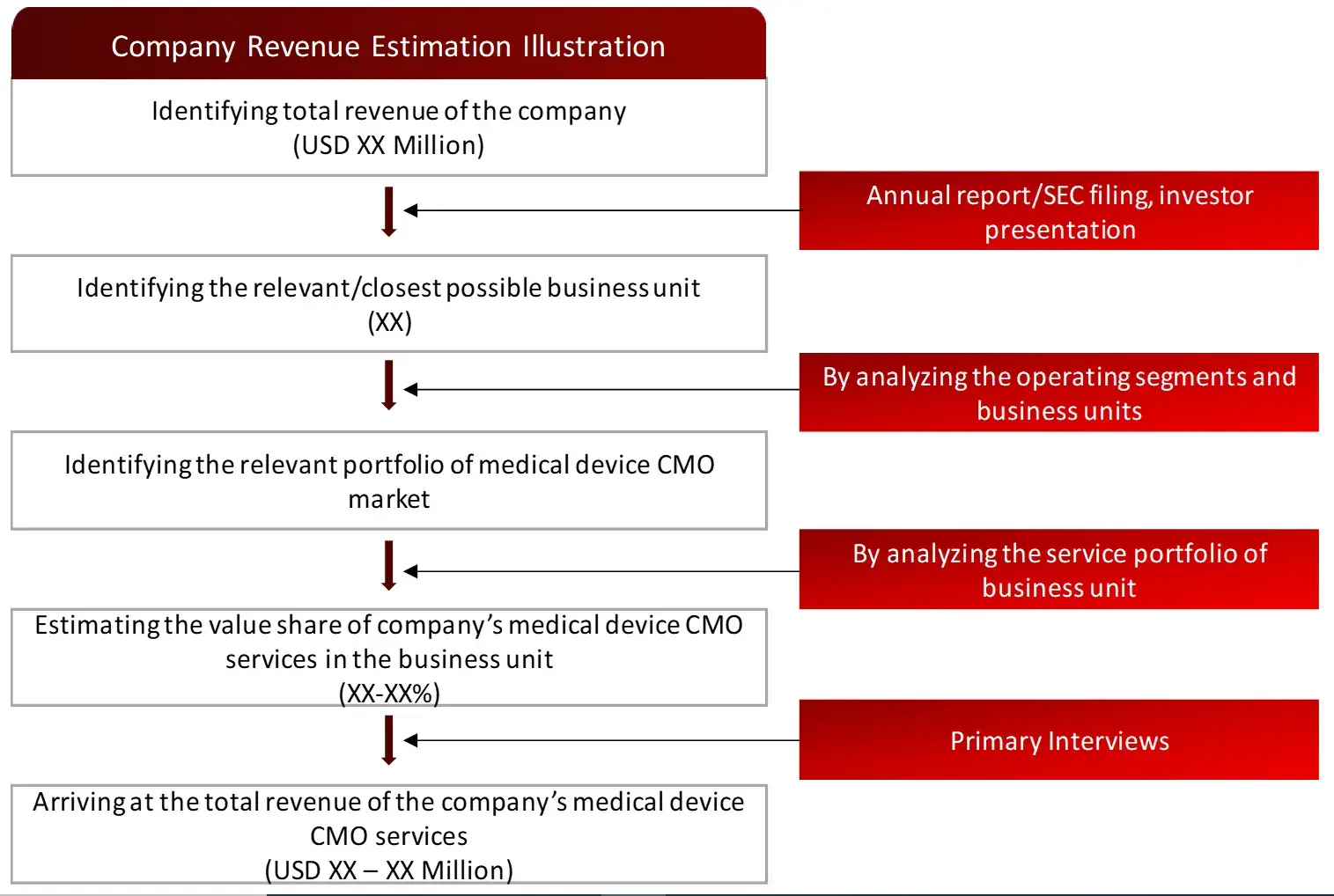 Sources: Company Websites, Annual Reports, SEC Filings, Press Releases, Investor Presentation, Paid Database, and Wissen Research Analysis.
Sources: Company Websites, Annual Reports, SEC Filings, Press Releases, Investor Presentation, Paid Database, and Wissen Research Analysis.FIGURE: REVENUE SHARE ANALYSIS OF KEY PLAYERS (SUPPLY SIDE)
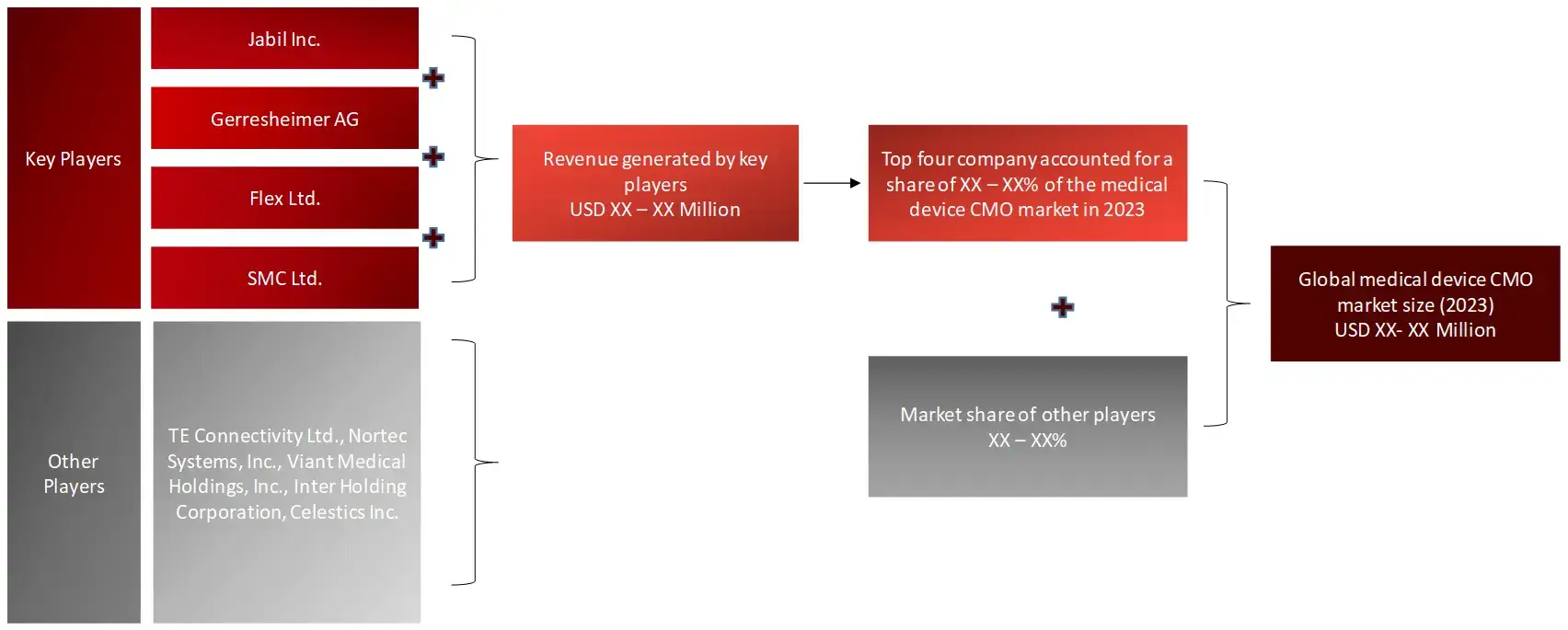
FIGURE: MARKET SIZE ESTIMATION TOP-DOWN AND BOTTOM-UP APPROACH
 Sources: Company Websites, Annual Reports, SEC Filings, Press Releases, Investor Presentation, Paid Database, and Wissen Research Analysis.
Sources: Company Websites, Annual Reports, SEC Filings, Press Releases, Investor Presentation, Paid Database, and Wissen Research Analysis.FIGURE: ANALYSIS OF DROCS FOR GROWTH FORECAST
 Sources: Medical Device Manufacturers Association (MDMA), Association of Indian Medical Device Industry (AIMED), Contract Manufacturers, Association of Medical Diagnostics Manufacturers (AMDM), MedTech Europe, National Health Institution (NIH), Company Website, Press Releases, Annual Reports, Paid Data Sources, and Wissen Research Analysis.
Sources: Medical Device Manufacturers Association (MDMA), Association of Indian Medical Device Industry (AIMED), Contract Manufacturers, Association of Medical Diagnostics Manufacturers (AMDM), MedTech Europe, National Health Institution (NIH), Company Website, Press Releases, Annual Reports, Paid Data Sources, and Wissen Research Analysis.FIGURE: GROWTH FORECAST ANALYSIS UTILIZING MULTIPLE PARAMETERS
 Sources: Company Websites, Annual Reports, SEC Filings, Press Releases, Investor Presentation, Paid Database, and Wissen Research Analysis.
Sources: Company Websites, Annual Reports, SEC Filings, Press Releases, Investor Presentation, Paid Database, and Wissen Research Analysis.Research Design
After arriving at the overall market size-using the market size estimation processes-the market will be split into several segments and sub segment. To complete the overall market engineering process and arrive at the exact statistics of each market segment and sub segment, the data triangulation, and market breakdown procedures will be employed, wherever applicable. The data will be triangulated by studying various factors and trends from both the demand and supply sides in the medical device CMO market.
 Sources: Medical Device Manufacturers Association (MDMA), Association of Indian Medical Device Industry (AIMED), Contract Manufacturers, Association of Medical Diagnostics Manufacturers (AMDM), MedTech Europe, National Health Institution (NIH), Company Website, Press Releases, Annual Reports, Paid Data Sources, and Wissen Research Analysis.
Sources: Medical Device Manufacturers Association (MDMA), Association of Indian Medical Device Industry (AIMED), Contract Manufacturers, Association of Medical Diagnostics Manufacturers (AMDM), MedTech Europe, National Health Institution (NIH), Company Website, Press Releases, Annual Reports, Paid Data Sources, and Wissen Research Analysis.1. Introduction
1.1 Key Objectives
1.2 Definitions
1.2.1 In Scope
1.2.2 Out of Scope
1.3 Scope of the Report
1.4 Scope Related Limitations
1.5 Key Stakeholders
2. Research Methodology
2.1 Research Approach
2.2 Research Methodology / Design
2.3 Market Sizing Approach
2.3.1 Secondary Research
2.3.2 Primary Research
3. Executive Summary & Premium Content
3.1 Global Market Outlook
3.2 Key Market Findings
4. Market Overview
4.1 Market Dynamics
4.1.1 Drivers/Opportunities
4.1.2 Restraints/Challenges
4.2 End User Perception
4.3 Need Gap
4.4 Supply Chain / Value Chain Analysis
4.5 Industry Trends
4.6 Porter’s Five Forces Analysis
5. Global Medical Device CMO Market, by Service Type (2023-2030, USD Million)
5.1 Medical Device Contract Manufacturing Services Market
5.1.1 Quality Management Services
5.1.1.1 Validation
5.1.1.2 Sterilization
5.1.2 Device Development Services
5.1.2.1 Development and Prototyping
5.1.2.2 Device / Component Manufacturing
5.1.2.3 Other Device Development Services (Verification etc.)
5.1.3 Packaging Services
5.1.3.1 Primary Packaging
5.1.3.2 Secondary Packaging
5.1.3.3 Labelling
5.1.4 Assembly Services
5.1.5 Other Services (Logistics and Warehousing)
6. Global Medical Device CMO Market, by Device Type (2023-2030, USD Million)
6.1 Diagnostic Devices
6.1.1 Consumables
6.1.2 Equipment
6.2 Therapeutic Devices
6.2.1 Cardiovascular Devices
6.2.2 Dental Devices
6.2.3 Diabetes Devices
6.2.4 Orthopedic Devices
6.2.5 Ophthalmology Devices
6.2.6 Respiratory Devices
6.2.7 Other Therapeutic Devices (Neurology, Urology etc.)
6.3 Drug Delivery Devices
6.3.1 Inhalation Drug Delivery Devices
6.3.2 Parenteral Drug Delivery Devices
6.3.2.1 Syringes
6.3.2.2 Pen Injectors
6.3.2.3 Auto Injectors
6.4 Other Devices (Pumps, Patched etc.)
7. Global Medical Device CMO Market, by Class (2023-2030, USD Million)
7.1 Class I
7.2 Class II
7.3 Class III
8. Global Medical Device CMO Market, by Target Application (2023-2030, USD Million)
8.1 Cardiovascular Devices
8.2 Diabetes Devices
8.3 Dental Devices
8.4 Orthopedic Devices
8.5 Ophthalmology Devices
8.6 Respiratory Devices
8.7 Surgical Devices
8.8 Other Applications (Urology, Neurology etc.)
9. Global Medical Device CMO Market, by Region (2023-2030, USD Million)
9.1 North America
9.1.1 US
9.1.2 Canada
9.2 Europe
9.2.1 Germany
9.2.2 France
9.2.3 Spain
9.2.4 Italy
9.2.5 UK
9.2.6 Rest of the Europe
9.3 Asia Pacific
9.3.1 China
9.3.2 Japan
9.3.3 India
9.3.4 Australia and New Zealand
9.3.5 South Korea
9.3.6 Rest of the Asia Pacific
9.4 Middle East and Africa
9.5 Latin America
10. Competitive Analysis
10.1 Key Players Footprint Analysis
10.2 Market Share Analysis
10.3 Key Brand Analysis
10.4 Regional Snapshot of Key Players
10.5 R&D Expenditure of Key Players
11. Company Profiles2
11.1 Jabil Inc.
11.1.1 Business Overview
11.1.2 Product Portfolio
11.1.3 Financial Snapshot3
11.1.4 Recent Developments
11.1.5 SWOT Analysis
11.2 Nipro Corporation
11.3 Flex Ltd.
11.4 Gerresheimer AG
11.5 Kimball Electronics Inc.
11.6 SMC Ltd.
11.7 Peter’s Technology Ltd.
11.8 Synecco
11.9 Celestica Inc.
11.10 Viant Medical Holdings, Inc.
11.11 Integer Holdings Corporation
11.12 TE Connectivity Ltd.
11.13 Nortec Systems, Inc.
12. Conclusion
13. Appendix
13.1 Industry Speak
13.2 Questionnaire
13.3 Available Custom Work
13.4 Adjacent Studies
13.5 Authors
14. References
Key Notes:
© Copyright 2024 – Wissen Research All Rights Reserved.