Netherton Syndrome: Treatment landscape- Market Insight, Clinical Trial, Product Analysis, Patent Analysis, Competitive Analysis and Market Forecast – 2023-2033
Netherton Syndrome, a rare genetic disorder affecting the skin, hair, and immune system, demands a nuanced and evolving treatment approach. This exploration delves into the multifaceted treatment landscape for managing the complex symptoms associated with this condition.
Foundational Therapies: The initial phase of treatment focuses on foundational measures. Emollients, keratolytics, and antibiotics play a crucial role in alleviating symptoms and preventing complications. Regular application of emollients helps maintain skin hydration, while keratolytics aid in managing excessive skin scaling. Antibiotics are essential to combat recurrent skin infections, a common challenge in Netherton syndrome.
Topical Steroids: For older children, cautiously administered topical steroids may offer relief from persistent pruritus and skin inflammation. However, their use in infants with erythroderma demands careful consideration due to potential complications such as pituitary adrenal axis suppression.
Emerging Therapies: Advancements in treatment options include innovative inhibitors like pimecrolimus, showing promise in managing skin involvement with low systemic absorption. Research into immunoglobulins and biologicals has demonstrated positive results, although the evidence remains in its early stages.
Cutting-edge Approaches: Exploring the frontier of treatments, gene therapy and epidermal stem cell therapy present exciting possibilities. These interventions aim to address the underlying genetic mutations responsible for Netherton syndrome, potentially offering more targeted and sustained relief.
Bacteriophage Preparations: A noteworthy development involves the use of antistaphylococcal bacteriophage preparations, particularly for patients allergic to antibiotics. This novel approach showcases significant improvement and holds promise for tailored interventions.
Phototherapies: Photochemotherapy (PUVA) and narrowband ultraviolet light B (NBUVB) have demonstrated efficacy in select cases. However, careful monitoring for the development of skin malignancies is imperative with these modalities.
Long-term Monitoring and Collaborative Care: Monitoring for associated conditions like trichorrhexis invaginata and ichthyosis linearis circumflexa is essential for early intervention. Collaborative care involving pediatricians, gastroenterologists, and geneticists ensures a comprehensive approach to managing this complex syndrome.
Conclusion: Netherton syndrome’s treatment landscape is evolving rapidly, embracing both conventional and cutting-edge interventions. As research progresses, a tailored, multidisciplinary approach offers hope for improved symptom management and an enhanced quality of life for those affected by this challenging genetic disorder.
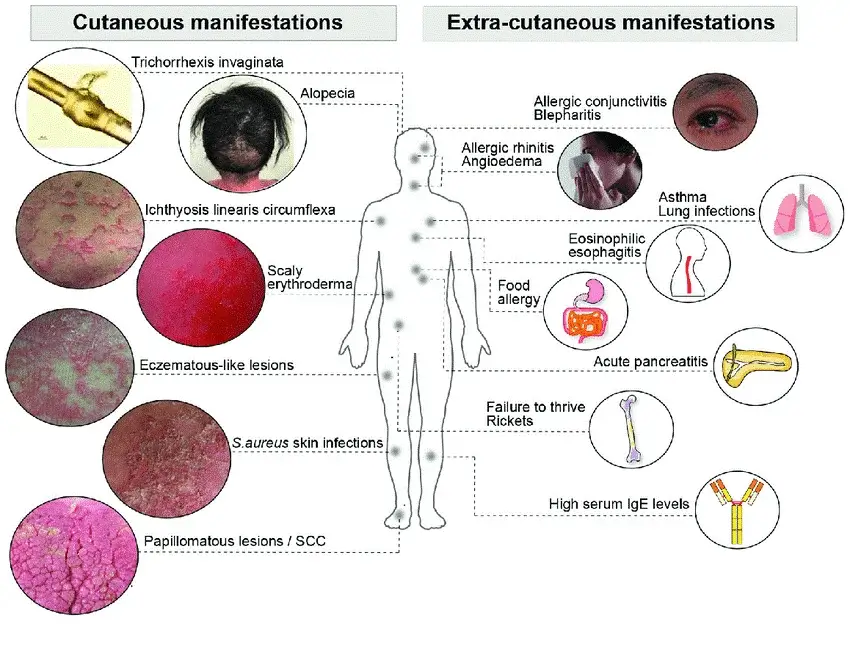
To attain a thorough comprehension of the dynamic terrain in Netherton Syndrome (NS) research and development, it is essential to delve into the present condition of scientific exploration and the emerging inventive therapeutic strategies. This understanding functions as a crucial guide for making knowledgeable decisions in the NS therapy realm, guaranteeing strategic selections and the efficient assimilation of therapies amid the constantly evolving healthcare scenario.
Market Landscape: Netherton Syndrome: Example Illustration: Distribution by Pipeline Candidates
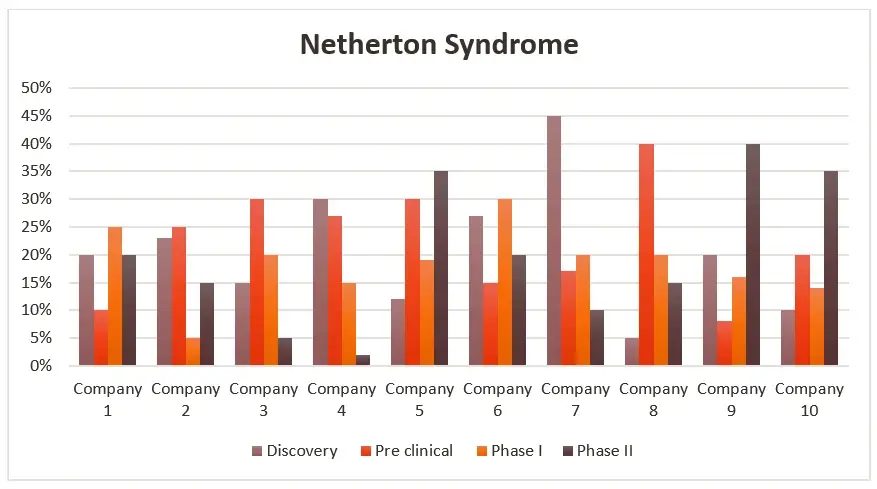
In the realm of Netherton Syndrome (NS) research and market analysis, company profiling assumes a pivotal role. It involves a meticulous scrutiny of firms involved in the NS sector, providing an all-encompassing perspective on a company’s historical context, array of products, financial standing, competitive strategies, and recent milestones. This valuable information facilitates the assessment of the competitive landscape within the NS market, as well as the identification of potential prospects for collaboration and strategic partnerships capable of fostering advancement and innovation in the field.
In this report, we will shall conduct a thorough screening of patents to comprehensively assess the intellectual property landscape within the Netherton Syndrome (NS) domain. The goal of this analysis is to uncover significant patents, identify influential inventors, and highlight emerging technological trends specific to the NS sector.
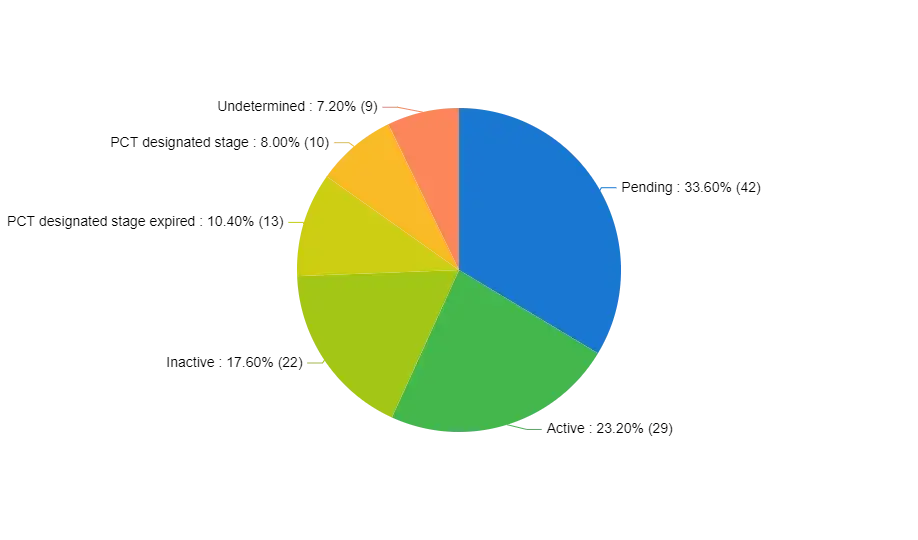

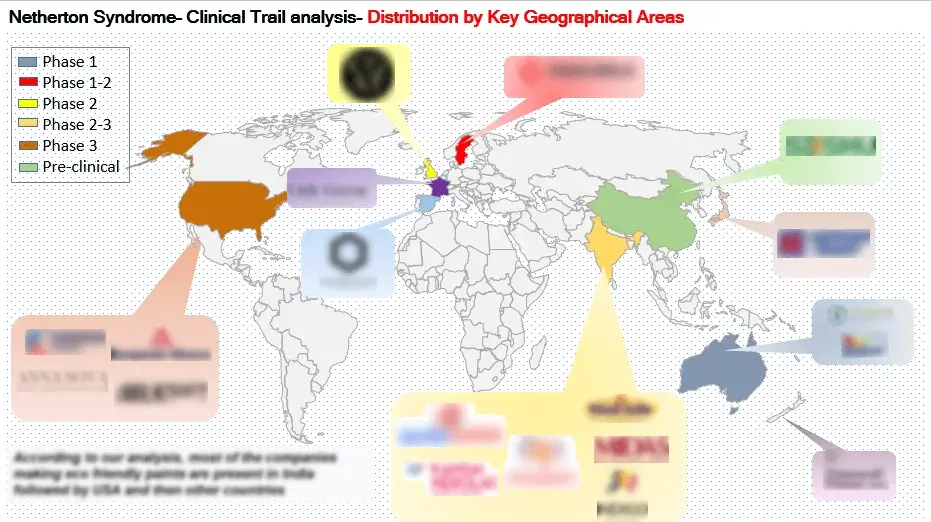
The clinical trial analysis segment within a market research report provides an in-depth assessment of clinical trials associated with Netherton Syndrome (NS). This involves a thorough breakdown of trial categories, study structures, research methodologies, and participant characteristics. Its primary objective is to evaluate the effectiveness, safety, and outcomes of pharmaceuticals and medical interventions within the context of NS.

This segment incorporates quantitative predictions and provides valuable insights into market size, revenue forecasts, and potential avenues for expansion within the NS domain.
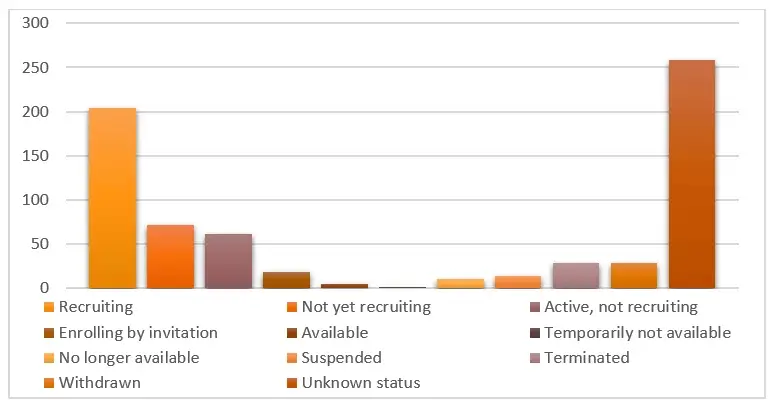
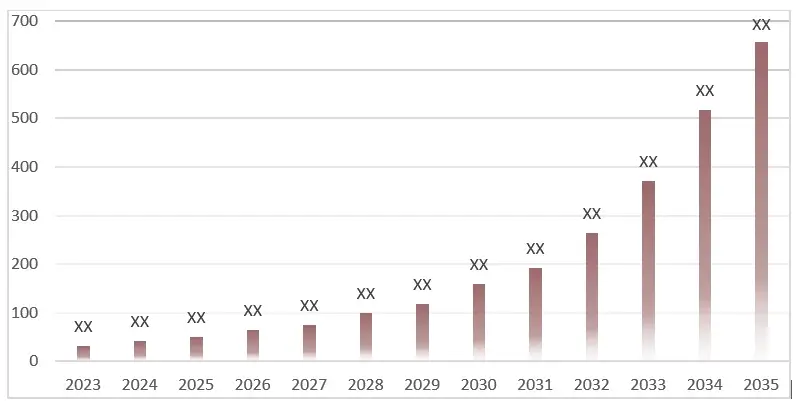
Within this market research report dedicated to Netherton Syndrome (NS), the section on the competitive landscape will deliver a concise overview of major market participants. It will include details on their individual market shares and present brief profiles outlining their strengths, weaknesses, and strategic approaches within the NS domain.
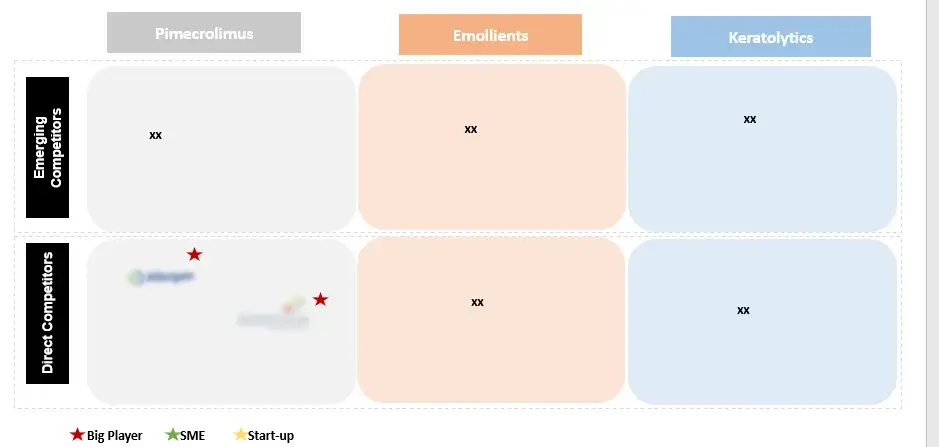
In order to give the most precise estimations and forecasts, Wissen Research uses an extensive and iterative research approach that is focused on reducing deviation. The company blends top-down and bottom-up methodologies for market segmentation and quantitative estimation. In addition, data triangulation, which examines the market from three separate angles, is a recurrent topic present in all of our research studies. Important components of the approach used for all of our studies include the following:
Preliminary data mining
On a wide scale, unprocessed market data is collected. Continuous data filtering makes sure that only verified and authenticated sources are taken into account. Additionally, data is extracted from a wide range of reports in our repository and from a number of reputable premium databases. We gather information from raw material suppliers, distributors, and purchasers to help with this since understanding the entire value chain is crucial for a thorough understanding of the market.
Surveys, technical symposia, and trade magazines are used to gather information on technical concerns and trends. Technical information focusing on white space and freedom of movement is also obtained from an intellectual property standpoint. Additionally, information on the industry’s drivers, constraints, and pricing patterns is obtained. As a result, a variety of original data are included in the material that is then cross-validated and certified with published sources.
Statistical model
We use simulation models to generate our market projections and estimates. Every study receives a special model that is tailored to it. Data for market dynamics, the technology environment, application development, and pricing patterns are gathered and supplied into the model all at once for analysis. The relative relevance of these factors is investigated, and their impact on the forecast period is assessed, using correlation, regression, and time series analysis. The process of market forecasting combines technological analysis with economic strategies, practical business acumen, and subject expertise.
Econometric models are frequently used for short-term forecasting, but technology market models are typically employed for long-term forecasting. These are based on a confluence of the business environment, regulatory environment, economic projection, and technical landscape. In order to develop global estimates, it is preferable to estimate markets from the bottom up by integrating data from key regional markets. This is required to ensure accuracy and a complete comprehension of the subject. Among the variables taken into account for forecasting are:
Regulations and anticipated developments
We give these criteria weights and use weighted average analysis to assess their market influence in order to calculate the anticipated market growth rate.
Primary research | Secondary research |
· Manufacturers · Technology distributors and wholesalers · End-user surveys · Consumer surveys | · Company reports and publications · Government publications · Independent investigations · Economic and demographic data · Online searches · Research reviews · Reference customers |
1. Executive Summary
1.1 Overview of Netherton Syndrome (NS)
1.2 Key Findings
1.3 Market Insights and Recommendations
2. Disease Overview
2.1 Definition and Classification of NS
2.2 Symptoms, Diagnosis, and Clinical Presentation
2.3 Pathophysiology and Genetic Basis
2.4 Epidemiology and Prevalence
2.5 Causes and Risk Factors
2.6 Treatment Options
3. Market Landscape
3.1 Market Size and Growth Trends, 2023-2033 (USD Million)
3.2 Market Segmentation (By Type, Treatment, Geography, etc.), 2023-2033 (USD Million)
3.3 Competitive Analysis of Key Players
3.4 Market Drivers and Challenges
4. Patent Analysis
4.1 Overview of NS Patents
4.2 Top Assignees
4.3 Geography Focus of Top Assignees
4.4 Legal Status
4.5 Network Analysis of Top Collaborating Entities in NS Treatments Patent Applications
4.6 Technology Evolution
4.7 Key Patents
4.8 Patent Trends and Innovations
4.9 Key Players and Patent Portfolio Analysis
5. Nethreton Syndrome Treatment Market: Revenue and Forecast to 2033 (USD Million)
5.1 Emollients and Keratolytics: Moisturizing Strategies
5.2 Antibiotics: Addressing Skin Infections
5.3 Topical Steroids: Considerations and Limitations
5.4 Topical Calcineurin Inhibitors (TCIs)
5.5 Alternative Treatments: PUVA (Photochemotherapy) and Oral Retinoids
6. Emerging Drugs
6.1 Key Cross Competition
6.2 QRX003: Quoin Pharmaceuticals
6.2.1 Drug Description
6.2.2 Other Developmental Activity
6.2.3 Clinical Development and Clinical Trials Information
6.2.4 Safety and Efficacy
6.2 SPEVIGO (spesolimab/BI 655130): Boehringer Ingelheim
6.2.1 Drug Description
6.2.2 Other Developmental Activity
6.2.3 Clinical Development and Clinical Trials Information
6.2.4 Safety and Efficacy
6.3 LM-030 (BPR277): LifeMax Laboratories/Novartis
6.3.1 Drug Description
6.3.2 Other Developmental Activity
6.3.3 Clinical Development and Clinical Trials Information
6.3.4 Safety and Efficacy
6.4 SXR1096: Sixera Pharma
6.4.1 Drug Description
6.4.2 Other Developmental Activity
6.4.3 Clinical Development and Clinical Trials Information
6.4.4 Safety and Efficacy
6.5 DS-2325: Daiichi Sankyo
6.5.1 Drug Description
6.5.2 Other Developmental Activity
6.5.3 Clinical Development and Clinical Trials Information
6.5.4 Safety and Efficacy
7. Clinical Trial Analysis
7.1 Chapter Overview
7.2 Analysis by Trial Registration Year
7.3 Analysis by Phase of Development
7.4 Analysis by Number of Patients Enrolled
7.5 Analysis by Status of Trial
7.6 Analysis by Study Design
7.8 Analysis by Geography
7.9 Analysis by Key Sponsors / Collaborators
8. Market Analysis by Region
8.1 North America
8.1.1 Total Market Size of NS in North America (2023-2033)
8.1.2 Market Size of NS by Therapies in North America (2023-2033)
8.2 Europe
8.2.1 Total Market Size of NS in Europe (2023-2033)
8.2.2 Market Size of NS by Therapies in Europe (2023-2033)
8.3 Asia-Pacific
8.3.1 Total Market Size of NS in Asia-Pacific (2023-2033)
8.3.2 Market Size of NS by Therapies in Asia-Pacific (2023-2033)
8.4 Latin America
8.4.1 Total Market Size of NS in Latin America (2023-2033)
8.4.2 Market Size of NS by Therapies in Latin America (2023-2033)
8.5 Middle East and Africa
8.5.1 Total Market Size of NS in Middle East and Africa (2023-2033)
8.5.2 Market Size of NS by Therapies in Middle East and Africa (2023-2033)
9. Future Outlook and Market Opportunities
9.1 Advancements in Research and Technology
9.2 Unmet Needs and Potential Market Gaps
9.3 Market Forecast and Growth Opportunities
10. Conclusion
11. Appendix
11.1 Glossary of Terms
11.2 List of Abbreviations
11.3 References
S.no | Key Highlights of Report | |
1. | Patent Analysis | · Top Assignee · Geography focus of top Assignees · Assignee Segmentation · Network analysis of the top collaborating entities in Netherton Syndrome therapy patent applications · Technology Evolution · Key Patents · Application and Issued Trend · Key technology |
2. | Market analysis | · Current Treatment Options · Emerging Therapies and Research Developments (by product analysis and scientific analysis) · Strategic activities · Therapeutic activity of drugs · Company portfolio Detailed profiles of the key players that are engaged in the development of approved drugs · |
3. | Clinical Trials | · Analysis of clinical trial through graphical representation · Coverage of treatments from pre-clinical phases till commercialization (also including terminated and completed studies) |
4. | Forecast | · Detailed comprehension of the historic, current and forecasted trend of market by analysis of impact of these treatments on the market |
5. | Opportunity Analysis | · Technology evolution based on problem solution · Potential licensees · Treatment trends · Unmet needs · SWOT · Drivers and barriers |
6. | KOLs | · A detailed analysis and identification of the key opinion leaders (KOLs), shortlisted based on their contributions |
LIST OF FIGURES
Figure number | Description |
Figure 1 | Terminology of Netherton Syndrome Over The Years |
Figure 2 | Netherton Syndrome Treatment– History and Present |
Figure 3 | Projection of Netherton Syndrome till 2033 in different geographies |
Figure 4 | Technology Categorization Of Drug Delivery Methods For Netherton Syndrome |
Figure 5 | Recent Technology Trends in Netherton Syndrome |
Figure 6 | Technology Evolution in Drug Delivery Market of Netherton Syndrome |
Figure 7 | Geographical Distribution of Patents of Top Assignees |
Figure 8 | Assignee Segmentation (Companies) |
Figure 9 | Assignee Segmentation (Educational Establishment) |
Figure 10 | Patent Based Key Insights of xx |
Figure 11 | Patent Based Key insights of xx |
Figure 12 | Patent Based Key insights of xx |
Figure 13 | Geographic Distribution of the Universities/Research Organizations Filling Patents On Various Drug Delivery Approaches |
Figure 14 | Key Summary Regarding the Patent Filing On Netherton Syndrome |
Figure 15 | Product Pipeline of Different Approaches with Companies Name |
Figure 16 | Portfolio for Approved Product |
Figure 17 | Clinical Trials Conducted till Date by Different Companies and Universities |
Figure 18 | Clinical Trials based Key Insights |
Figure 19 | Key Growth Drivers for Netherton Syndrome Market |
Figure 20 | Restraints for Netherton Syndrome Market |
Figure 21 | xx Portfolio (Top Player) |
Figure 22 | xx Portfolio (Top Player) |
Figure 23 | xx Portfolio (Top Player) |
Figure 24 | xx Portfolio (Top Player) |
Figure 25 | xx Portfolio (Top Player) |
Figure 26 | xx Portfolio (Start-up) |
Figure 27 | xx Portfolio (Start-up) |
Figure 28 | xx Portfolio (Start-up) |
Figure 29 | Strategic Activities Including Collaboration, Partnerships and Acquisitions |
Figure 30 | Research Methodology for Patent, Selection and Analysis |
Figure 31 | Research Methodology for Clinical Trials, Selection and Analysis |
LIST OF GRAPHS
Graph number |
Description |
Graph 1 | Number of people worldwide with Netherton Syndrome |
Graph 2 | Problem Solution Analysis |
Graph 3 | Top Assignees in Netherton Syndrome |
Graph 4 | Technology Focus of Top Assignees (IPC-CPC Classes) |
Graph 5 | Top Countries of Origin of Patents |
Graph 6 | New entrants in drug delivery field |
Graph 7 | Legal Status |
Graph 8 | Most Cited Patents |
Graph 9 | Patents with Largest Invention Families |
Graph 10 | Most Claim-Heavy Patents |
Graph 11 | Filing Trends |
Graph 12 | Clinical Trial Filing Timeline |
Graph 13 | Recruitment Status of the Clinical Trials Related to the Different Drug Delivery Approaches |
Graph 14 | Clinical Trials Phases with Respect to Specific Drug Delivery Approach |
Graph 15 | Weighted Scores for Top Players According to Benchmarking Criteria |
Graph 16 | Netherton Syndrome (CAGR: 2023-2033) |
Graph 17 | Netherton Syndrome Market Share: Distribution by Key Geographical Area, 2023-2033 |
LIST OF TABLES
Table number | Description |
Table 1 | Parameters included and excluded for conducting the analysis |
Table 2 | Technology Classes with Definitions |
Table 3 | Patent Litigation |
Table 4 | Highest Market Valued Patents |
Table 5 | SWOT Analysis of Top 3 Players |
Table 6 | Parameters and their score for Benchmarking |
Table 7 | Weighted scores for top 5 players according to benchmarking criteria |
© Copyright 2024 – Wissen Research All Rights Reserved.