Vaccines: Market Size, Trend, by Application (Pneumococcal Disease, Influenza, Combination Vaccines, Polio etc.) Technology (Conjugate Vaccines, Recombinant Vaccines, Inactivated & Subunit Vaccines, Viral Vector Vaccines, mRNA Vaccines, etc.) End User, Region, Major Players – Global Forecast to 2030
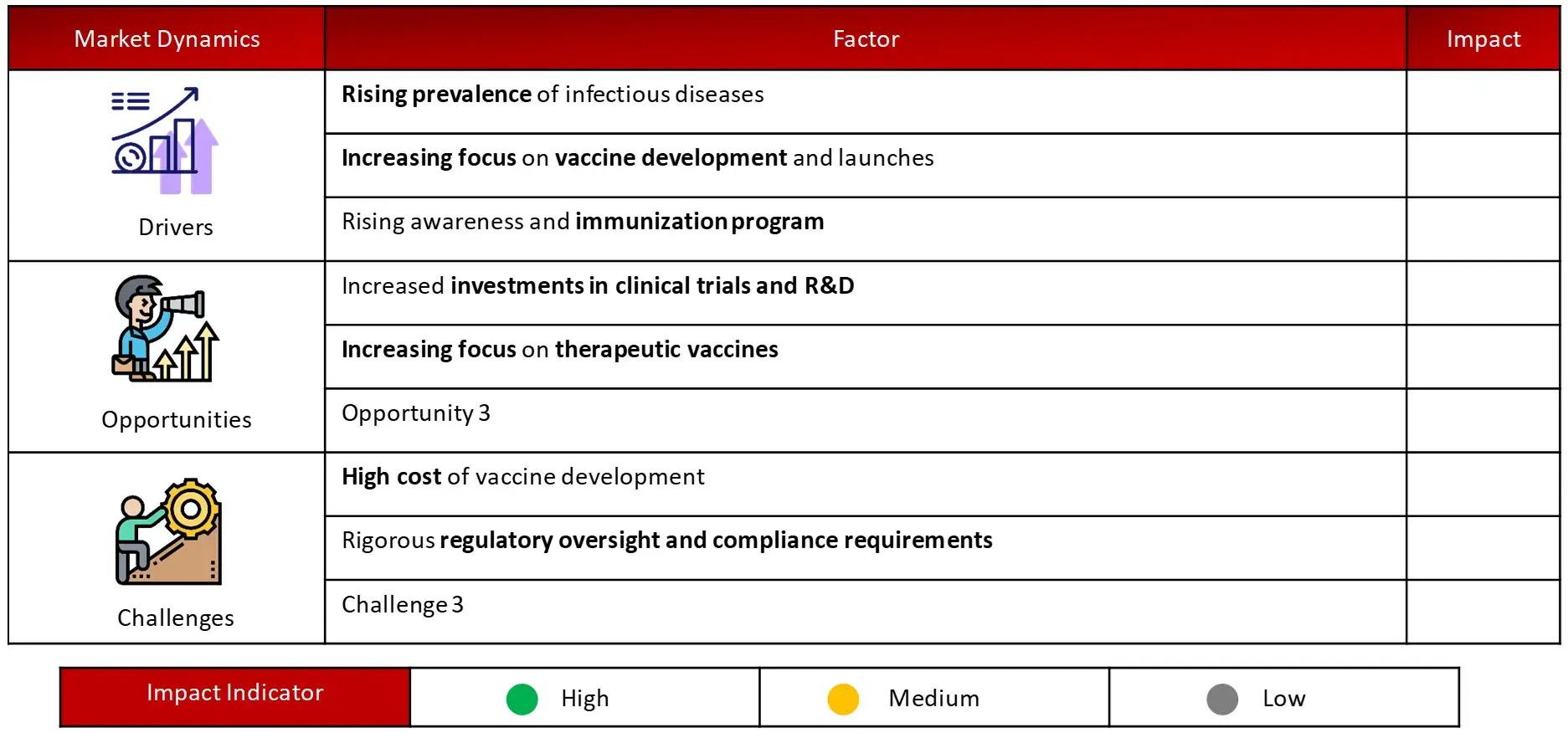
Key driving factors of vaccines market include rising prevalence of infectious disease, increasing focus on vaccine development and launches and rising awareness and immunization program
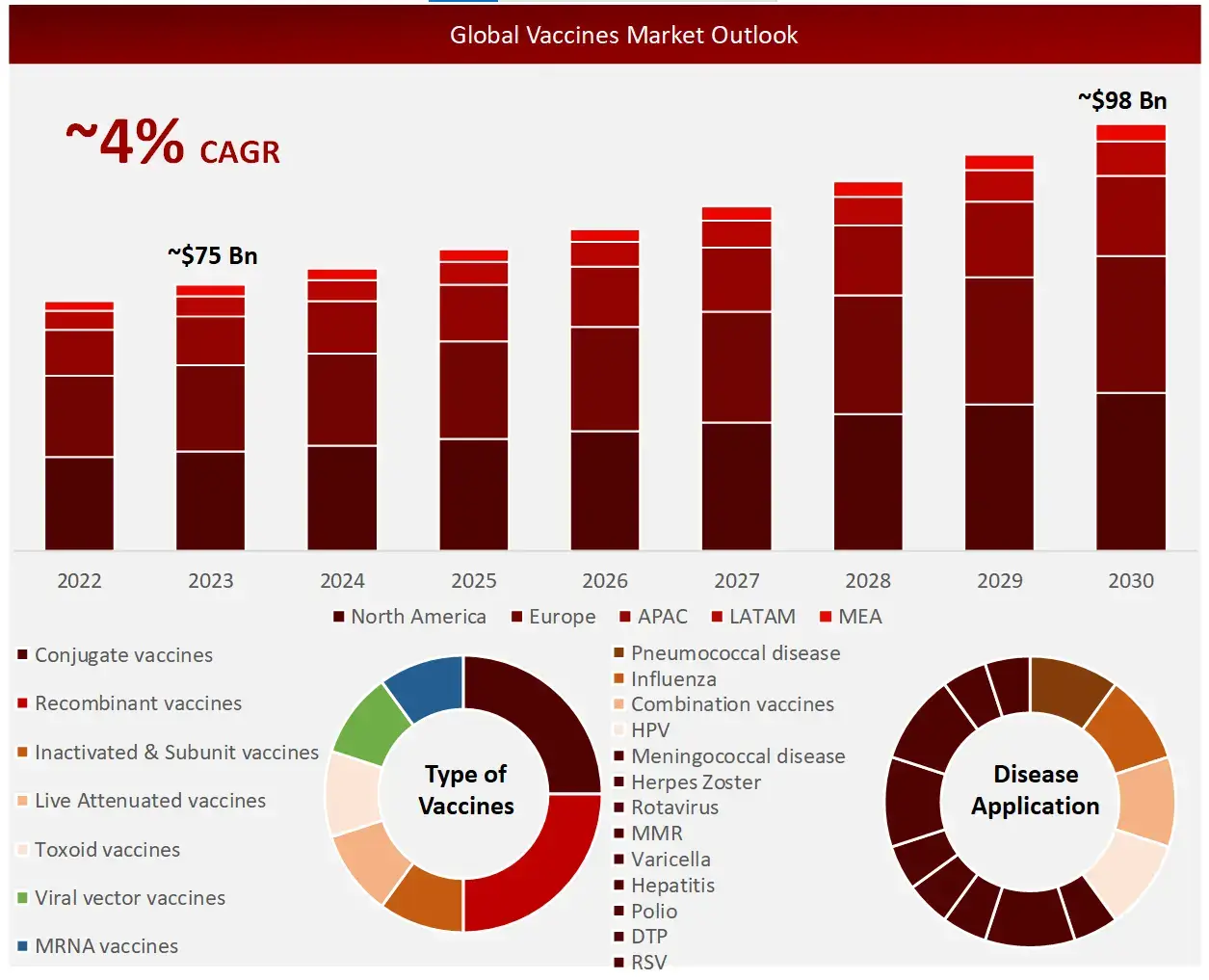
Challenges in the vaccines domain include high cost of vaccine development and rigorous regulatory oversight. The global vaccines market is estimated to be valued at ~ $75 Billion in 2023, and is expected to grow at a CAGR of ~4%. Conjugate vaccines have held the most significant share in the market since 2022, with North America dominating the regional market share.
Key players functioning in vaccines sector are GSK plc, Johnsons & Johnsons, Merck, AstraZeneca, Pfizer and CSL. In recent strategic activities, GSK paid 400 Million Euros to CureVac to acquires full rights to develop, manufacture and commercialise globally mRNA candidate vaccines for influenza and COVID-19, including combinations. (July, 2024) and collaboration between Sanofi vaccines and Medidata, to deploy Medidata’s eCOA for vaccine studies. (Feb, 2024)
The vaccines market is experiencing substantial growth, driven by increasing awareness and widespread immunization programs. Global health initiatives have significantly boosted vaccine uptake, with the World Health Organization reporting that immunization prevents 4-5 million deaths annually. In 2022, the Global Vaccine Action Plan achieved 85% coverage for essential vaccines in over 125 countries.
Furthermore, public health campaigns have enhanced awareness, leading to higher vaccination rates. For instance, a 2023 study across 30 countries found that targeted awareness programs increased flu vaccination rates by 23% among high-risk populations. The COVID-19 pandemic has also heightened public understanding of vaccine importance, with over 13 billion doses administered globally as of 2023, demonstrating the critical role of immunization in public health and driving market expansion.
Major driving opportunity in vaccines market is due to increased investments in clinical trials and R&D. According to Wissen Research’s 2023 study, global pharmaceutical companies allocated over $35 billion to vaccine research, a ~40% increase from 2019 levels. This surge in funding has accelerated the development of novel vaccines, with over 250 candidates in various stages of clinical trials as of 2024.
Notably, mRNA technology, spotlighted during the COVID-19 pandemic, has opened new avenues for vaccine development across multiple diseases. A recent industry report revealed that 60% of major vaccine manufacturers have expanded their R&D facilities in the past two years. Additionally, public-private partnerships have flourished, with governments worldwide pledging $10 billion for vaccine research initiatives in 2023 alone. This influx of resources is driving innovation, potentially revolutionizing disease prevention and expanding the vaccine market’s scope.
High development costs can impede innovation and market growth. Developing a new vaccine typically costs between an estimate $200 million to $500 million, with some estimates reaching as high as $1 billion. This substantial investment is exacerbated by lengthy development timelines, often spanning 10-15 years from concept to market.
Furthermore, the complex manufacturing processes and stringent quality control measures add to the overall expense. For instance, a recent industry study by Wissen Research revealed that 40% of vaccine manufacturers reported a 30% increase in production costs over the past five years due to enhanced safety protocols and advanced technologies. These high costs can deter smaller companies from entering the market and limit the development of vaccines for less common diseases.
Conjugate vaccines dominated the vaccines market share in 2023
Vaccines include various technology types, including conjugate vaccines, recombinant vaccines, inactivated & subunit vaccines, live attenuated vaccines, toxoid vaccines, viral vector vaccines, MRNA vaccines, among others. Each category serves specific therapeutic needs across different disease applications. Among these, conjugate vaccines have held the largest market share, primarily due to the high global demand.
Covid-19 vaccines accounted for the largest share in 2023
Global vaccines market by application is segmented into a diverse indication spectrum, some of them are Covid-19, polio, rota virus, influenza, HPV, herpes zoster and others.
Among the aforementioned applications covid-19 segment dominated the market due to ongoing vaccination drives globally, to eradicate this viral disease.
Significant market share held by hospitals was observed in 2022
Vaccines serve a number of end-users such as hospitals, ambulatory facilities, vaccination centers.
The traditional healthcare facilities, i.e. hospitals remained the significant holder of user market share due to increase in the immunization drives globally, such as for Covid-19.
North America held the largest market share in vaccines in the forecast period
Vaccines market research included a comprehensive analysis of five key regions:
All regions were evaluated based on these following factors- healthcare infrastructure, regulatory landscape, technological adoption, and market dynamics.
The research observed that North America held the largest share in the vaccines domain during the forecast period, primarily due to its advanced healthcare systems, high adoption rates, and the presence of major market players driving innovation in this sector.
As per the historical and the base year of the report (2022 and 2023, respectively), key players in vaccines were GSK plc (UK), Merck & Co (Germany), Pfizer (US), CSL (Australia), Sanofi (France), Johnson & Johnson (US), Emergent (US), AstraZeneca (UK), Serum Institute of India (India), Bavarian Nordic (Denmark), Daiichi Sankyo Company (Japan), Panacea Biotech (India), Bharat Biotech (India), Novavax (US) and Inovio Pharmaceutical (US).
Introduction
Market Definition
Vaccines are a biological preparation that is used to stimulate body immune response against disease. This preparation consists of suspensions of weakened or killed microorganisms or toxins or other biological mixtures, such as those containing antibodies, lymphocytes or mRNA, administered primarily to prevent diseases.
FIGURE: VACCINES EQUIPMENT MARKET SEGMENT
Sources: Company Websites and Wissen Research Analysis
FIGURE: YEARS FRAMEWORK CONSIDERED IN THE STUDY
Sources: Wissen Research Analysis
Key Stakeholders
Key objectives of the Study
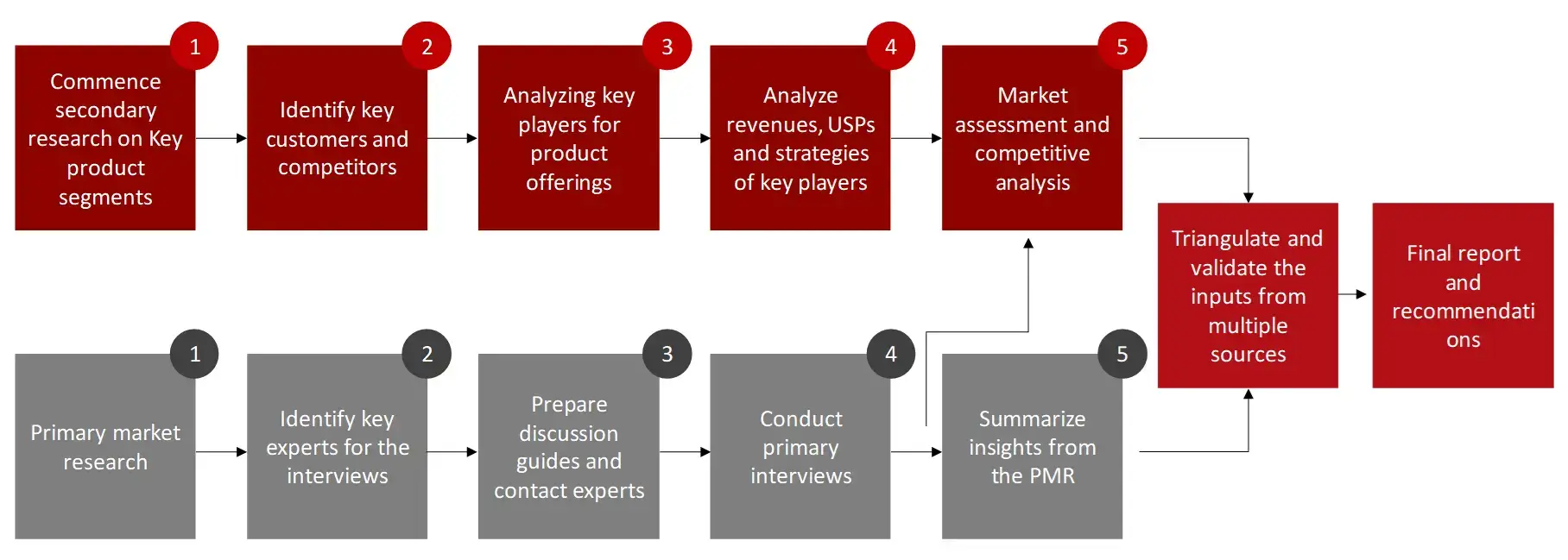
Research Methodology
The objective of the study is to analyze the key market dynamics such as drivers, opportunities, challenges, restraints, and key player strategies. To track company developments such as product launches and approvals, expansions, and collaborations of the leading players, the competitive landscape of the vaccines market to analyze market players on various parameters within the broad categories of business and product strategy. Top-down and bottom-up approaches will be used to estimate the market size. To estimate the market size of segments and sub segments the market breakdown and data triangulation will be used.
FIGURE: RESEARCH DESIGN
Sources: Wissen Research Analysis
Research Approach
Collecting Secondary Data
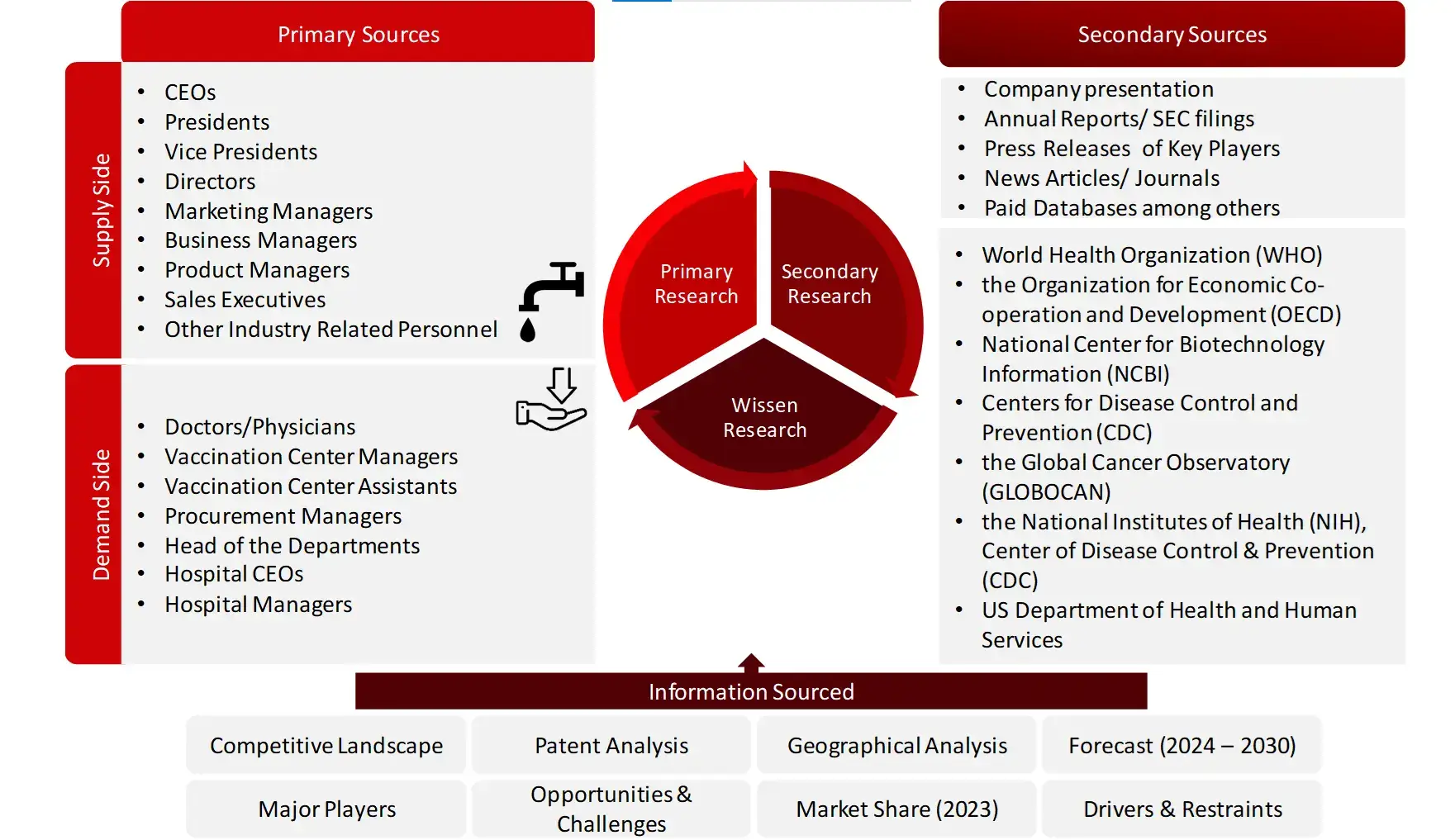
The secondary research data collection process involves the usage of secondary sources, directories, databases, annual reports, investor presentations, and SEC filings of companies. Secondary research will be used to identify and collect information useful for the extensive, technical, market-oriented, and commercial study of the vaccines market. A database of the key industry leaders will also be prepared using secondary research.
Collecting Primary Data
The primary research data will be conducted after acquiring knowledge about the vaccines market scenario through secondary research. A significant number of primary interviews will be conducted with stakeholders from both the demand side and supply side (including various industry experts, such as Directors, Chief X Officers (CXOs), Vice Presidents (VPs) from business development, marketing and product development teams, product manufacturers) across major countries of North America, Europe, Asia Pacific, and Rest of the World. Primary data for this report was collected through questionnaires, emails, and telephonic interviews.
FIGURE: BREAKDOWN OF PRIMARY INTERVIEWS FROM SUPPLY SIDE
FIGURE: BREAKDOWN OF PRIMARY INTERVIEWS FROM DEMAND SIDE
FIGURE: PROPOSED PRIMARY PARTICIPANTS FROM DEMAND AND SUPPLY SIDE
Note: Above mentioned companies are non- exhaustive
Market Size Estimation
All major manufacturers offering various vaccines will be identified at the global/regional level. Revenue mapping will be done for the major players, which will further be extrapolated to arrive at the global market value of each type of segment. The market value of vaccines market will also split into various segments and sub segments at the region level based on:
FIGURE: REVENUE MAPPING BY COMPANY (ILLUSTRATION)
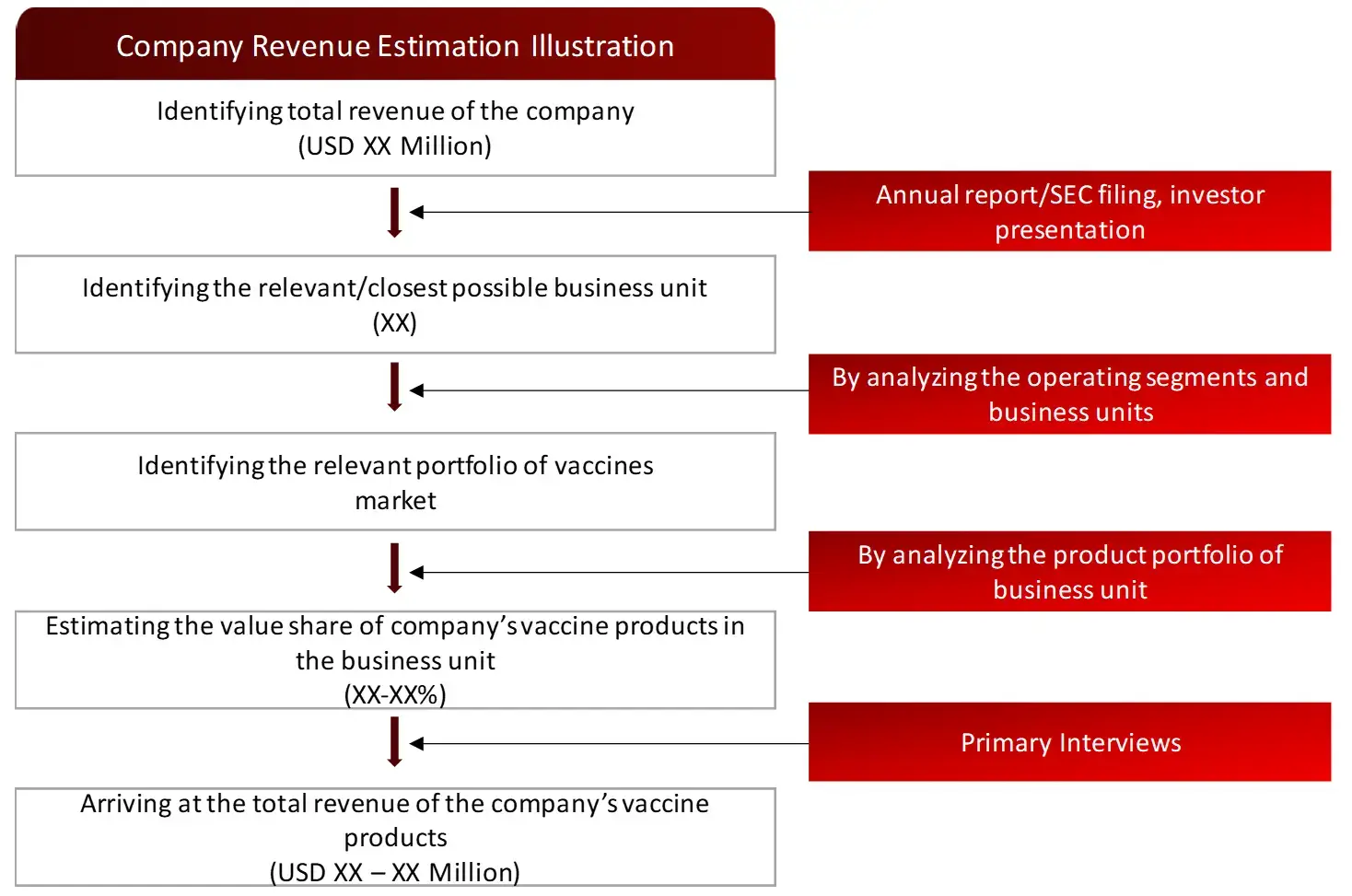
FIGURE: REVENUE SHARE ANALYSIS OF KEY PLAYERS (SUPPLY SIDE)
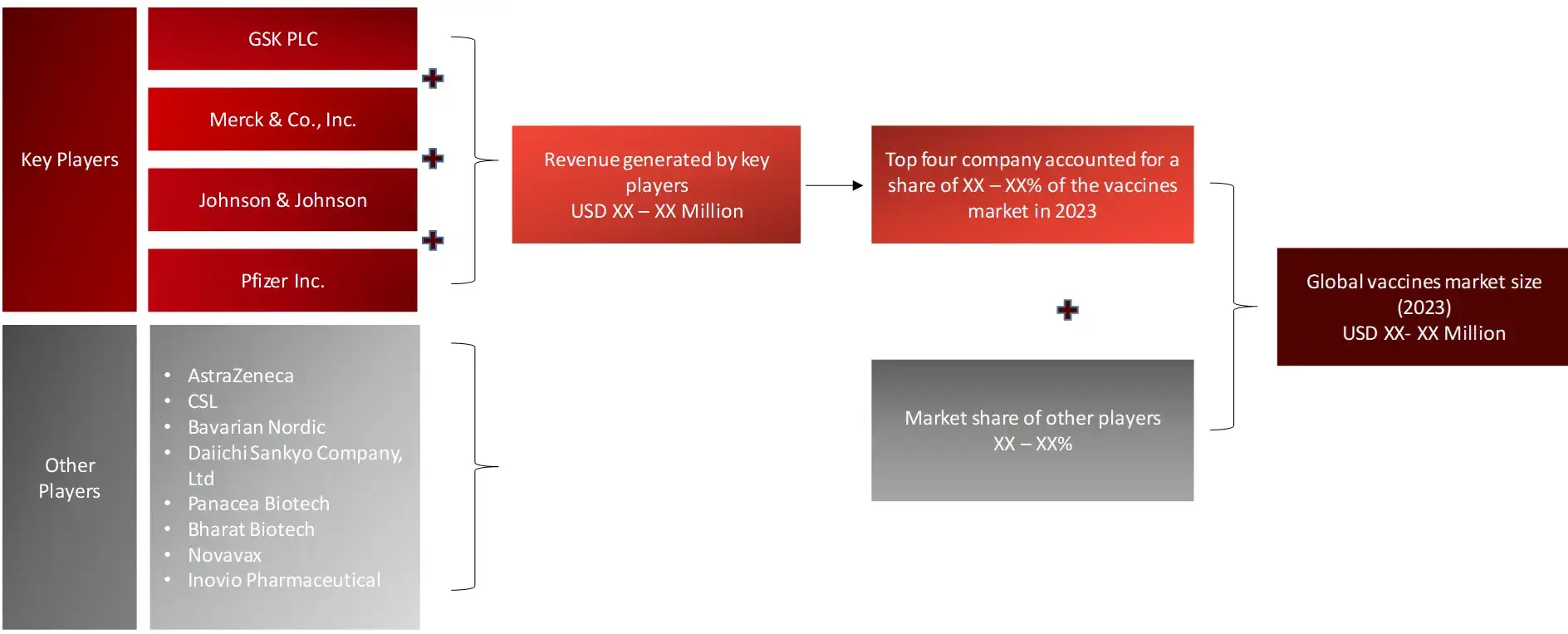
FIGURE: MARKET SIZE ESTIMATION TOP-DOWN AND BOTTOM-UP APPROACH
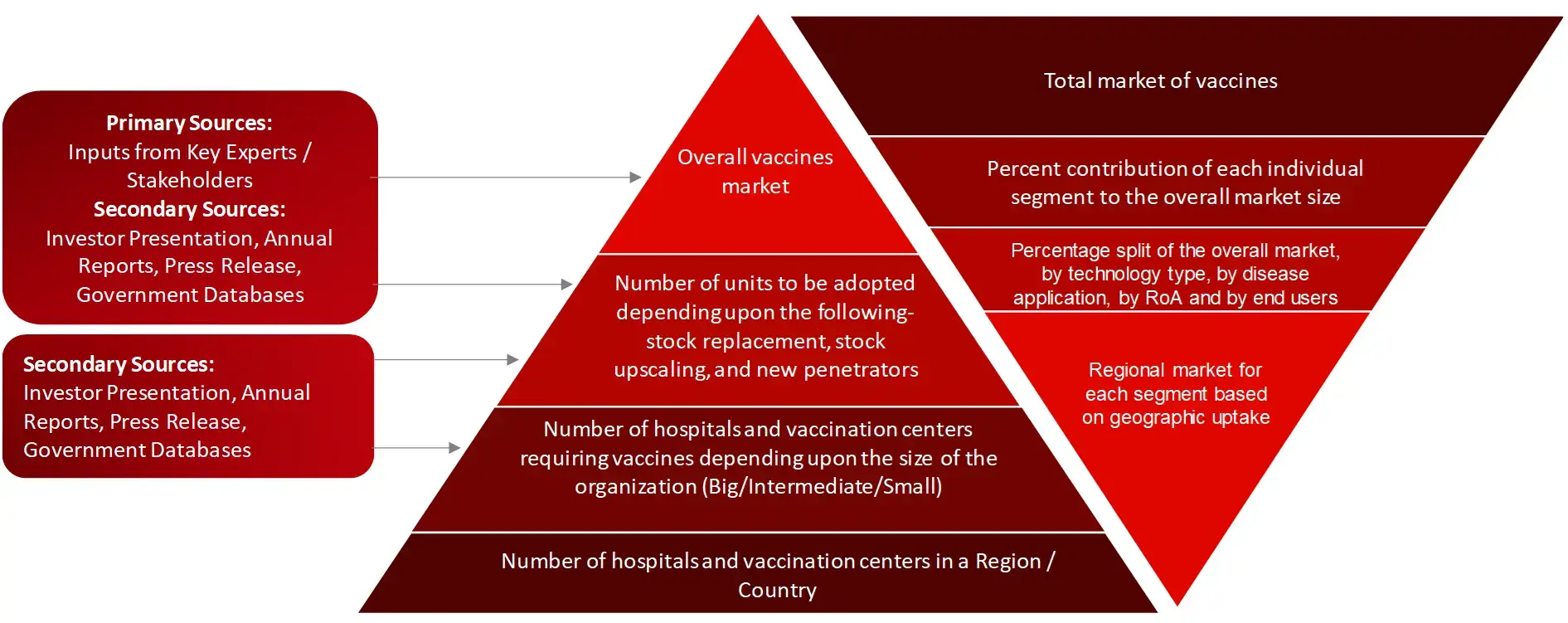
FIGURE: ANALYSIS OF DROCS FOR GROWTH FORECAST
Sources: World Health Organization (WHO), the Organization for Economic Co-operation and Development (OECD), National Center for Biotechnology Information (NCBI), Centers for Disease Control and Prevention (CDC), the Global Cancer Observatory (GLOBOCAN), the National Institutes of Health (NIH), Center of Disease Control & Prevention (CDC), US Department of Health and Human Services, National Institutes of Health (NIH), National Library of Medicine, National Center for Biotechnology Information (NCBI), National Institute of Allergy and Infectious Diseases (NIAID), World Cancer Research Fund International (WCRF International), European Medicines Agency (EMA), The National Medical Products Administration (NMPA), Global Alliance for Vaccines and Immunization (GAVI), United States Food & Drug Administration (US FDA), Orange book, Purple book, Clinical trials.gov, Pan American Health Organization (PAHO), United Nation International Children’s Emergency Fund (UNICEF), Department of health and Human Services (HHS), International Society for Vaccines (ISV). The Central Drugs Standard Control Organization (CDSCO)
FIGURE: GROWTH FORECAST ANALYSIS UTILIZING MULTIPLE PARAMETERS
Research Design
After arriving at the overall market size-using the market size estimation processes-the market will be split into several segments and sub segment. To complete the overall market engineering process and arrive at the exact statistics of each market segment and sub segment, the data triangulation, and market breakdown procedures will be employed, wherever applicable. The data will be triangulated by studying various factors and trends from both the demand and supply sides in the vaccines market industry.
1. Introduction
1.1 Key objectives
1.2 Definitions
1.2.1 In scope
1.2.2 Out of scope
1.3 Scope of the report
1.4 Scope related limitations
1.5 Key stakeholders
2. Research Methodology
2.1 Research approach
2.2 Research methodology / design
2.3 Market sizing approach
2.3.1 Secondary research
2.3.2 Primary research
3. Executive Summary & Premium Content
3.1 Global market outlook
3.2 Key market findings
4.Market Overview
4.1 Market dynamics
4.1.1 Drivers/Opportunities
4.1.2 Restraints/Challenges
4.2 End user perception
4.3 Need gap
4.4 Supply chain / Value chain analysis
4.5 Industry trends
4.6 Porter’s Five Forces analysis
4.7 Pricing analysis
4.8 Reimbursement Scenario
5. Patent Analysis
5.1 Top Assignees in Vaccines Market
5.2 Geography Focus of Top Assignees
5.3 Legal Status of Vaccines Patents
5.4 Assignee Segmentation
5.5 Network Analysis of Top Collaborating Entities in Vaccines Patent Applications
5.6 Technology Evolution in Vaccines
5.7 Key Patents in Vaccines
5.8 Patent Trends and Innovations
5.9 Key Players and Patent Portfolio Analysis
6. Clinical Trial Analysis
6.1 Overview of vaccine clinical trials
6.2 Analysis by trial registration year
6.3 Analysis by phase of development
6.4 Analysis by number of patients enrolled
6.5 Analysis by status of trial
6.6 Analysis by study design
6.7 Analysis by intervention type
6.8 Analysis by geography
6.9 Analysis by key sponsors/collaborators
7. Vaccines Market, by Technology (2023-2030, USD Million)
7.1 Conjugate vaccines
7.2 Recombinant vaccines
7.3 Inactivated & Subunit vaccines
7.4 Live Attenuated vaccines
7.5 Toxoid vaccines
7.6 Viral vector vaccines
7.7 MRNA vaccines
7.8 Others
8. Vaccines Market, by Type (2023-2030, USD Million)
8.1 Multivalent vaccines
8.2 Monovalent vaccines
9. Vaccines Market, by Disease Application (2023-2030, USD Million)
9.1 Pneumococcal disease
9.2 Influenza
9.3 Combination vaccines
9.4 HPV
9.5 Meningococcal disease
9.6 Herpes Zoster
9.7 Rotavirus
9.8 MMR (measles, mumps and rubella)
9.9 Varicella
9.10 Hepatitis
9.11 DTP
9.12 Polio
9.13 RSV (respiratory syncytial virus)
9.14 Others
10. Vaccines Market, by Route of Administration (2023-2030, USD Million)
10.1 Intramuscular & subcutaneous administration
10.2 Oral administration
10.3 Others
11. Vaccines Market, by End Users (2023-2030, USD Million)
11.1 Hospitals and clinics
11.2 Ambulatory care centres
11.3 Vaccination centres
11.4 Others
12. Vaccines Market, by Age Group (2023-2030, USD Million)
12.1 Adult vaccines
12.2 Pediatric vaccines
13. Vaccines Market, by Region (2023-2030, USD Million)
13.1 North America
13.1.1 US
13.1.2 Canada
13.2 Europe
13.2.1 UK
13.2.2 France
13.2.3 Germany
13.2.4 Italy
13.2.5 Spain
13.2.6 Rest of Europe
13.3 Asia Pacific
13.3.1 China
13.3.2 India
13.3.3 Japan
13.3.4 South Korea
13.3.5 Australia and New Zealand
13.3.6 Rest of Asia Pacific
13.4 Middle East and Africa
13.5 Latin America
14. Competitive Analysis
14.1 Key player’s footprint analysis
14.2 Market share analysis
14.3 Key brand analysis
14.4 Regional snapshot of key players
14.5 R&D expenditure of key players
15. Company Profiles2
15.1 GSK PLC
15.1.1 Business overview
15.1.2 Product portfolio
15.1.3 Financial snapshot3
15.1.4 Recent developments
15.1.5 SWOT analysis
15.2 Merck & Co., Inc.
15.3 Pfizer Inc.
15.4 CSL
15.5 Sanofi
15.6 Johnson & Johnson, Inc.
15.7 Emergent
15.8 AstraZeneca
15.9 Serum Institute of India Pvt., Ltd.
15.10 Bavarian Nordic
15.11 Daiichi Sankyo Company, Ltd
15.12 Panacea Biotech
15.13 Bharat Biotech
15.14 Novavax
15.15 Inovio Pharmaceutical
15.16 Others Key Players
16. Appendix
16.1 Industry speak
16.2 Questionnaire
16.3 Available custom work
16.4 Adjacent studies
16.5 Authors
17. References
Key Notes:
© Copyright 2024 – Wissen Research All Rights Reserved.